Top Qs
Timeline
Chat
Perspective
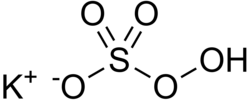
Potassium peroxymonosulfate
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Potassium peroxymonosulfate, also referred to as potassium peroxysulfate and potassium monopersulfate (KMPS), is an inorganic compound with the formula KHSO5. It is the mono-potassium salt derived from peroxymonosulfuric acid (Caro's acid). It is a constituent of the widely used oxidizing agent called Oxone, which is a triple salt with the formula 2KHSO5·KHSO4·K2SO4.[2][3][4]
Remove ads
Related salts
Organic-soluble derivatives of peroxymonosulfate include the tetra-n-butylammonium, tetraphenylphosphonium, and benzyltriphenylphosphonium salts: (nBu4N)HSO5, (Ph4P)HSO5, and (BnPh3P)HSO5.[5][6] The ammonium and sodium salts of HSO−5 are also known.
Applications
The title compound is the active ingredient in oxone, which is a common disinfectant[7] and whitening agent.[2] It has also been investigated for use in processes aimed at delignification of wood.[8]
Underlying these uses is the high redox potential, which for potassium peroxymonosulfate, per se, is +1.81 V.
Structure
The structure of the monohydrate has been confirmed by X-ray crystallography. This analysis reveals the expected tetrahedral sulfur center, an O-O bond length of 146 picometers, and an SOOH dihedral angle of 90°.[9]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads