Top Qs
Timeline
Chat
Perspective
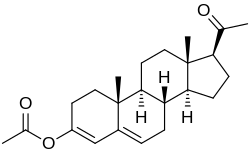
Progesterone 3-acetyl enol ether
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Progesterone 3-acetyl enol ether, also known as progesterone acetate,[1] as well as 3-acetoxypregna-3,5-dien-20-one, is a progestin which was never marketed.[2][3][4][5] It was reported to possess similar potency to progesterone and hydroxyprogesterone caproate in the rabbit endometrial carbonic anhydrase test, a bioassay of progestogenic activity.[2][3] In addition, it was able to maintain pregnancy in animals.[2] Progesterone 3-acetyl enol ether is closely related to quingestrone, which is also known as progesterone 3-cyclopentyl enol ether and was formerly marketed as an oral contraceptive.[6]
The 3-acetyl ether may be cleaved from progesterone 3-acetyl enol ether in vivo and, based on its chemical structure, this may result in the transformation of progesterone 3-acetyl enol ether into 3α-dihydroprogesterone and/or 3β-dihydroprogesterone. 3β-Dihydroprogesterone has been reported to possess about the same progestogenic potency as progesterone in the Clauberg test, whereas 3α-dihydroprogesterone was not assessed.[7]
The C3 enol ethers of progesterone are less suited for use via depot injection relative to progestogen esters like hydroxyprogesterone caproate due to their susceptibility to oxidative metabolism.[8]
Remove ads
See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads