Top Qs
Timeline
Chat
Perspective
Pyrosulfate
From Wikipedia, the free encyclopedia
Remove ads
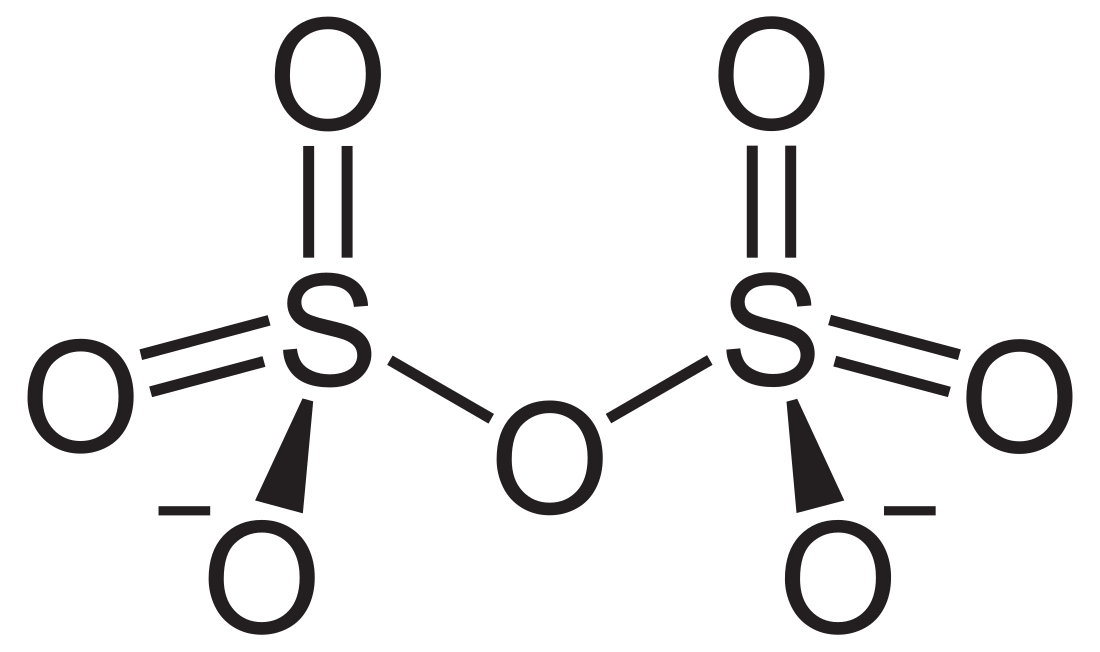
In chemistry, disulfate or pyrosulfate is the anion with the molecular formula S
2O2−
7. Disulfate is the IUPAC name. [1]
It has a dichromate-like structure and can be visualised as two corner-sharing SO4 tetrahedra, with a bridging oxygen atom.[2]
In this anion, sulfur has an oxidation state of +6. Disulfate is the conjugate base of the hydrogen disulfate (hydrogen pyrosulfate) ion HS
2O−
7, which in turn is the conjugate base of disulfuric acid (pyrosulfuric acid).

Remove ads
Role in sulfation
Industrial production of sulfate ester-based surfactants involves the reaction (sulfation) of fatty alcohols with sulfur trioxide. For example, dodecyl alcohol is sulfated using sulfur trioxide. The reaction proceeds by initial formation of the pyrosulfate:
- 2 SO3 + ROH → ROSO2−O−SO3H
- ROSO2−O−SO3H → ROSO3H + SO3
Several million tons are produced annually.[3]
See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads