Top Qs
Timeline
Chat
Perspective
Ro60-0175
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
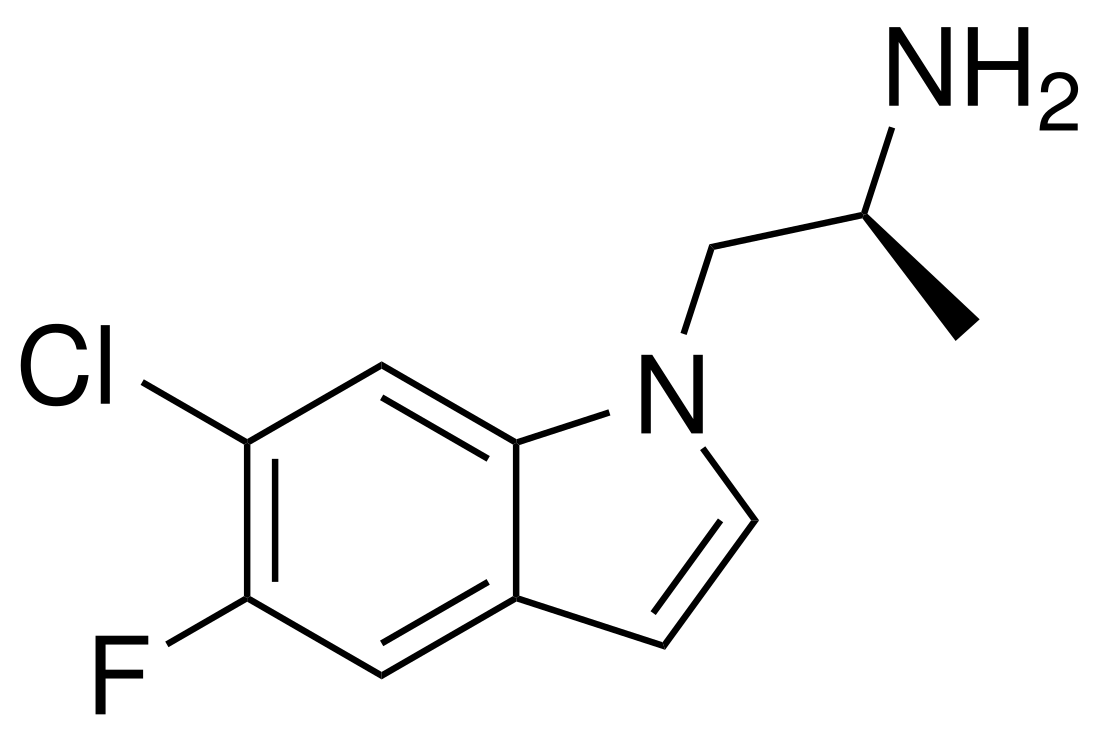
Ro60-0175, or Ro-600175, also known as (S)-5-fluoro-6-chloro-α-methylisotryptamine ((S)-5-F-6-Cl-isoAMT), is a serotonin 5-HT2 receptor agonist of the isotryptamine family developed by Hoffmann–La Roche, which has applications in scientific research.[1][2][3] It is the enantiopure (S)- isomer of the 5-fluoro and 6-chloro derivative of α-methylisotryptamine (isoAMT).[1]
It acts as a potent and selective agonist of both the serotonin 5-HT2B and 5-HT2C receptor subtypes, with good selectivity over the closely related serotonin 5-HT2A subtype, and little or no affinity at other receptors.[4][5] However, Ro60-0175 also activates the serotonin 5-HT2A receptor less potently than the serotonin 5-HT2B and 5-HT2C receptors.[6] Its EC50 and Emax values have been found to be 0.91–2.4 nM (79–130%) at the serotonin 5-HT2B receptor, 32–52 nM (84–88%) at the serotonin 5-HT2C receptor, and 400–447 nM (69–91%) at the serotonin 5-HT2A receptor.[1]
The drug has been found to produce hypolocomotion and sedative-like effects,[7] antidepressant-like effects,[8] anxiolytic-like effects[9] or no change in anxiety-like responses,[7][10] anti-obsessive-like effects,[10] antipsychotic-like effects,[10] appetite suppression,[11] and penile erections in rodent animal studies.[12] It fully generalizes with the preferential serotonin 5-HT2C receptor agonist meta-chlorophenylpiperazine (mCPP) in rodent drug discrimination tests, which can be blocked by the selective serotonin 5-HT2C receptor antagonist SB-242084.[13] Ro60-0175 also generalizes with the selective serotonin reuptake inhibitor (SSRI) citalopram in rodent drug discrimination tests, which can likewise be blocked by SB-242084, suggesting a major role for the serotonin 5-HT2C receptor in the interoceptive effects of SSRIs.[14] The drug has been found to inhibit dopaminergic signaling in the mesolimbic pathway.[15][16]
Ro60-0175 does not induce the head-twitch response, a behavioral proxy of psychedelic effects, when administered alone in rodents.[17][6] In addition, it suppresses the head-twitch response induced by the psychedelic drug (R)-DOI.[18][19] However, in combination with the selective serotonin 5-HT2C receptor antagonist SB-242084, Ro60-0175 robustly induces the head-twitch response.[17][6] This effect is abolished by addition of the selective serotonin 5-HT2A receptor antagonist ketanserin or volinanserin.[6] The preceding findings suggest that Ro60-0175 may be a serotonergic psychedelic and may have hallucinogenic effects in humans at sufficiently high doses or in combination with a serotonin 5-HT2C receptor antagonist.[6]
The drug was first described in the scientific literature by 1996.[8] It was under development by Roche for the treatment of major depressive disorder (MDD), anxiety disorders, and obsessive–compulsive disorder (OCD), and reached the preclinical research stage of development, but development was discontinued in 1997.[20][21]
Remove ads
See also
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads