Top Qs
Timeline
Chat
Perspective
Samarium(II) chloride
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Samarium(II) chloride (SmCl2) is a chemical compound, used as a radical generating agent in the ketone-mediated intraannulation reaction.
Remove ads
Preparation
Reduction of samarium(III) chloride with samarium metal in a vacuum at a temperature of 800 °C to 900 °C, or with hydrogen gas at 350 °C yields samarium(II) chloride:[1]
- 2 SmCl3 + Sm → 3 SmCl2
- 2 SmCl3 + H2 → 2 SmCl2 + 2 HCl
Samarium(II) chloride can also be prepared by reducing samarium(III) chloride with lithium metal/naphthalene in THF:[3]
- SmCl3 + Li → SmCl2 + LiCl
A similar reaction has been observed with sodium.[2]
Remove ads
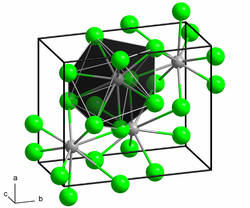
Structure
Samarium(II) chloride adopts the PbCl2 (cotunnite) structure.[2]
Physical properties
The compound co-crystallises with fermium dichloride (FmCl2).[4]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads