Top Qs
Timeline
Chat
Perspective
Scandium nitride
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
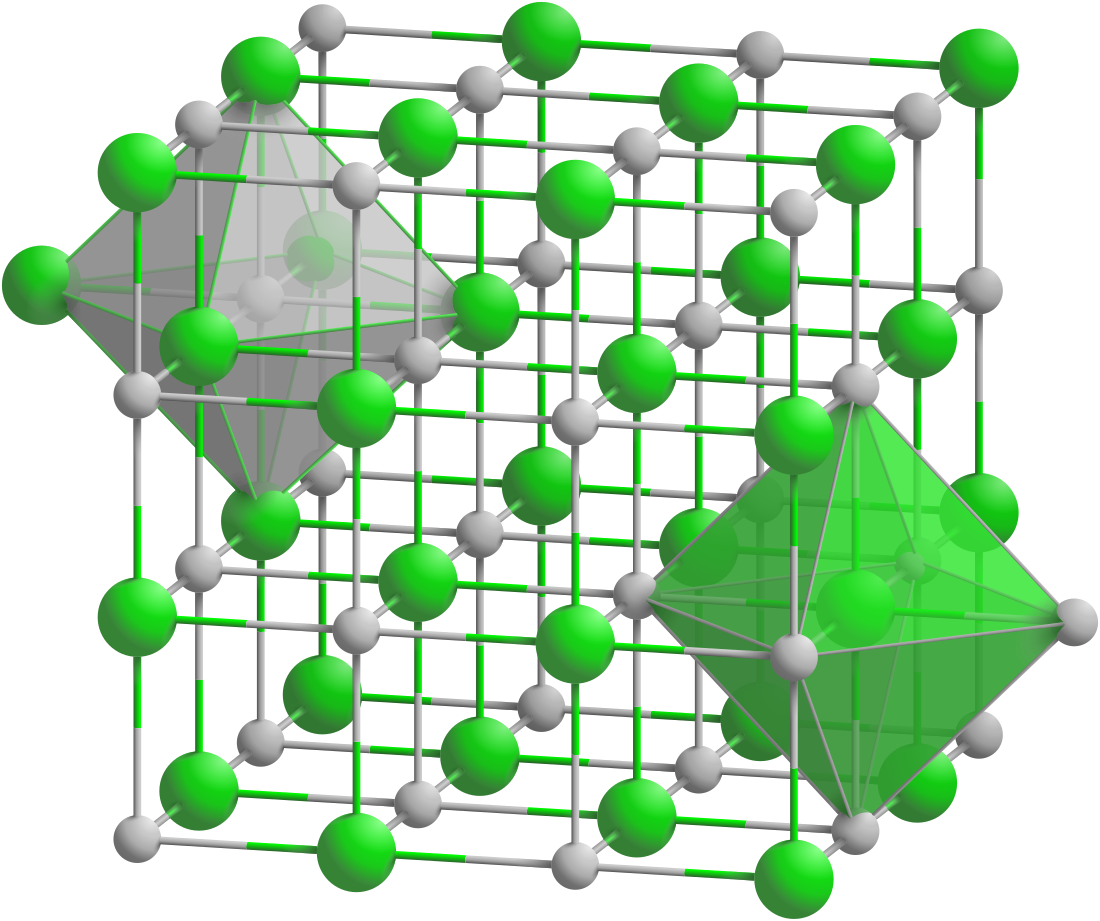
Scandium nitride (ScN) is a binary III-V indirect bandgap semiconductor. It is composed of the scandium cation and the nitride anion. It forms crystals that can be grown on tungsten foil through sublimation and recondensation.[1] It has a rock-salt crystal structure with octahedral bonding coordination. It exhibits lattice constant of 0.451 nm and an indirect bandgap of 0.9 eV and direct bandgap of 2 to 2.4 eV.[1][2] These crystals can be synthesized by dissolving nitrogen gas with indium-scandium melts, magnetron sputtering, Molecular Beam Epitaxy (MBE), HVPE and other deposition methods.[2][3] Scandium nitride is also an effective gate for semiconductors on a silicon dioxide (SiO2) or hafnium dioxide (HfO2) substrate.[4] Scandium nitride is the first nitride semiconductor reported to be synthesized without an active Nitrogen plasma source using the Molecular Beam Epitaxy (MBE) technique. It exhibits a scavenging effect, in which scandium at the growth front dissociates molecular nitrogen and incorporates it into the lattice.[5] Scandium nitride can be potentially used in thermoelectric materials as a semiconducting layer in epitaxial single-crystalline metal/semiconductor superlattices for thermoelectric, plasmonic and thermophotonic applications, and as a substrate material for high-quality GaN-based devices and other solid-state applications.[6]
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads