Top Qs
Timeline
Chat
Perspective
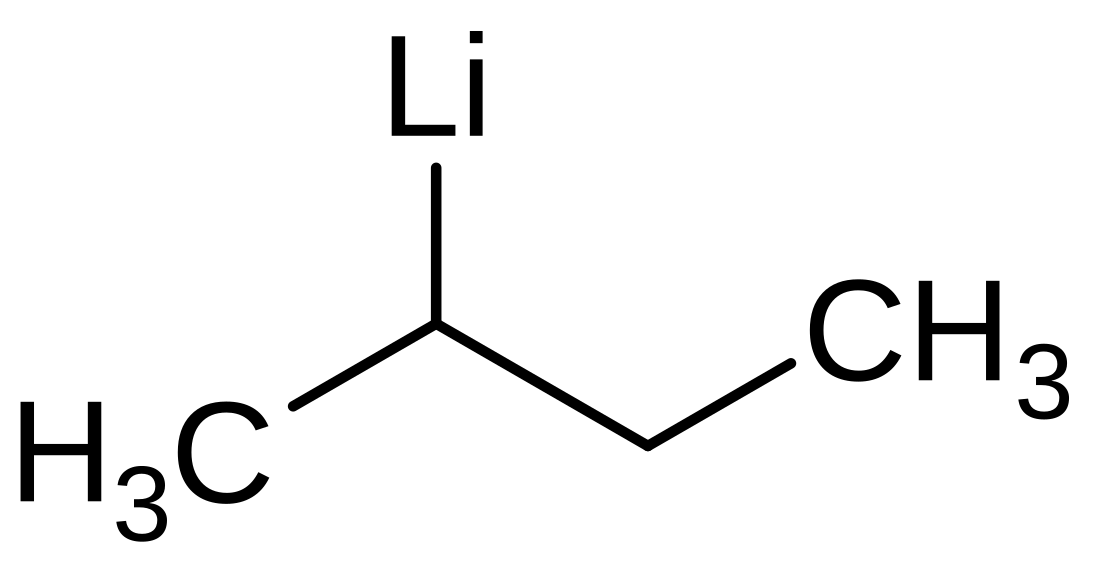
Sec-Butyllithium
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
sec-Butyllithium is an organometallic compound with the formula CH3CHLiCH2CH3, abbreviated sec-BuLi or s-BuLi. This chiral organolithium reagent is used as a source of sec-butyl carbanion in organic synthesis.[2]
Remove ads
Synthesis
sec-BuLi can be prepared by the reaction of sec-butyl halides with lithium metal:[3]
Properties
Summarize
Perspective
Physical properties
sec-Butyllithium is a colorless viscous liquid.[2][4] Using mass spectrometry, it was determined that the pure compound has a tetrameric structure.[5] It also exists as tetramers when dissolved in organic solvents such as benzene, cyclohexane or cyclopentane.[4] The cyclopentane solution has been detected with 6Li-NMR spectroscopy to have a hexameric structure at temperatures below −41 °C.[6] In electron-donating solvents such as tetrahydrofuran, there exists an equilibrium between monomeric and dimeric forms.[7]
Chemical properties
The carbon-lithium bond is highly polar, rendering the carbon basic, as in other organolithium reagents. Sec-butyllithium is more basic than the primary organolithium reagent, n-butyllithium. It is also more sterically hindered. sec-BuLi is employed for deprotonations of particularly weak carbon acids where the more conventional reagent n-BuLi is unsatisfactory. It is, however, so basic that its use requires greater care than for n-BuLi. For example diethyl ether is attacked by sec-BuLi at room temperature in minutes, whereas ether solutions of n-BuLi are stable.[2]
The compound decomposes slowly at room temperature and more rapidly at higher temperatures, giving lithium hydride and a mixture of butenes.[8][9]
Remove ads
Applications
Many transformations involving sec-butyllithium are similar to those involving other organolithium reagents.
In combination with sparteine as a chiral auxiliary, sec-butyllithium is useful in enantioselective deprototonations.[10] It is also effective for lithiation of arenes.[11]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads