Top Qs
Timeline
Chat
Perspective
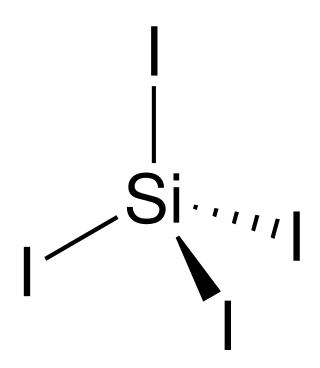
Silicon tetraiodide
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Silicon tetraiodide is the chemical compound with the formula SiI4. It is a tetrahedral molecule with Si-I bond lengths of 2.432(5) Å.[1]
SiI4 is a precursor to silicon amides of the formula Si(NR2)4 (R = alkyl).[2] It has also been of interest in the manufacture and etching of silicon in microelectronics.
Remove ads
Synthesis and reactions
This compound is produced by treating silicon-copper mixture with iodine:[3]
- Si + I2 → SiI4
It reacts quickly with water and moisture in the air.
It can also be made on a large scale by reaction of silicon or silicon carbide with iodine on heating to about 200 °C. Of more academic interest is the reaction of silane with iodine vapour at 130 - 150 °C, as this produces a series of compounds ranging from iodosilane SiH3I to diiodosilane SiH2I2 and triiodosilane SiHI3 as well. These compounds are colourless liquids at room temperature.[4] The last one can be readily distinguished from the similar carbon compound, iodoform which is a yellow solid at room temperature.
Remove ads
Comparison with other SiX4 compounds
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads