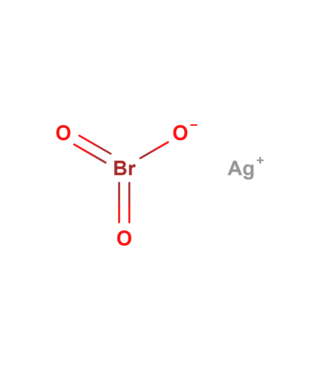
Silver bromate is an inorganic compound with the molecular formula AgBrO3. It is a white powder that is toxic and is both light and heat-sensitive.[2]
Quick facts Names, Identifiers ...
Silver bromate
 |
| Names |
| Systematic IUPAC name
|
| Other names
Argentous bromate |
| Identifiers |
|
|
|
|
| ChemSpider |
|
| ECHA InfoCard |
100.029.120 |
|
|
|
|
InChI=1S/Ag.BrHO3/c;2-1(3)4/h;(H,2,3,4)/q+1;/p-1 Y Key: XQLMNMQWVCXIKR-UHFFFAOYSA-M Y InChI=1/Ag.BrHO3/c;2-1(3)4/h;(H,2,3,4)/q+1;/p-1 Key: XQLMNMQWVCXIKR-REWHXWOFAZ
|
|
| Properties |
|
AgBrO3 |
| Molar mass |
235.770 g/mol |
| Appearance |
white powder
photosensitive |
| Density |
5.206 g/cm3 |
| Melting point |
309 °C (588 °F; 582 K) |
|
0.167 g/100 mL |
|
5.38×10−5[1] |
| Solubility in ammonium hydroxide |
soluble |
| Hazards |
| GHS labelling: |
|
  |
|
Danger |
|
H272, H315, H319, H335 |
|
P210, P220, P261, P264, P271, P280, P302+P352, P304+P340+P312, P305+P351+P338, P332+P313, P337+P313, P362+P364, P370+P378, P403+P233, P405, P501 |
| Safety data sheet (SDS) |
MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). |
Close
 Silver bromide GIF
Silver bromide GIF