Top Qs
Timeline
Chat
Perspective
Stibole
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
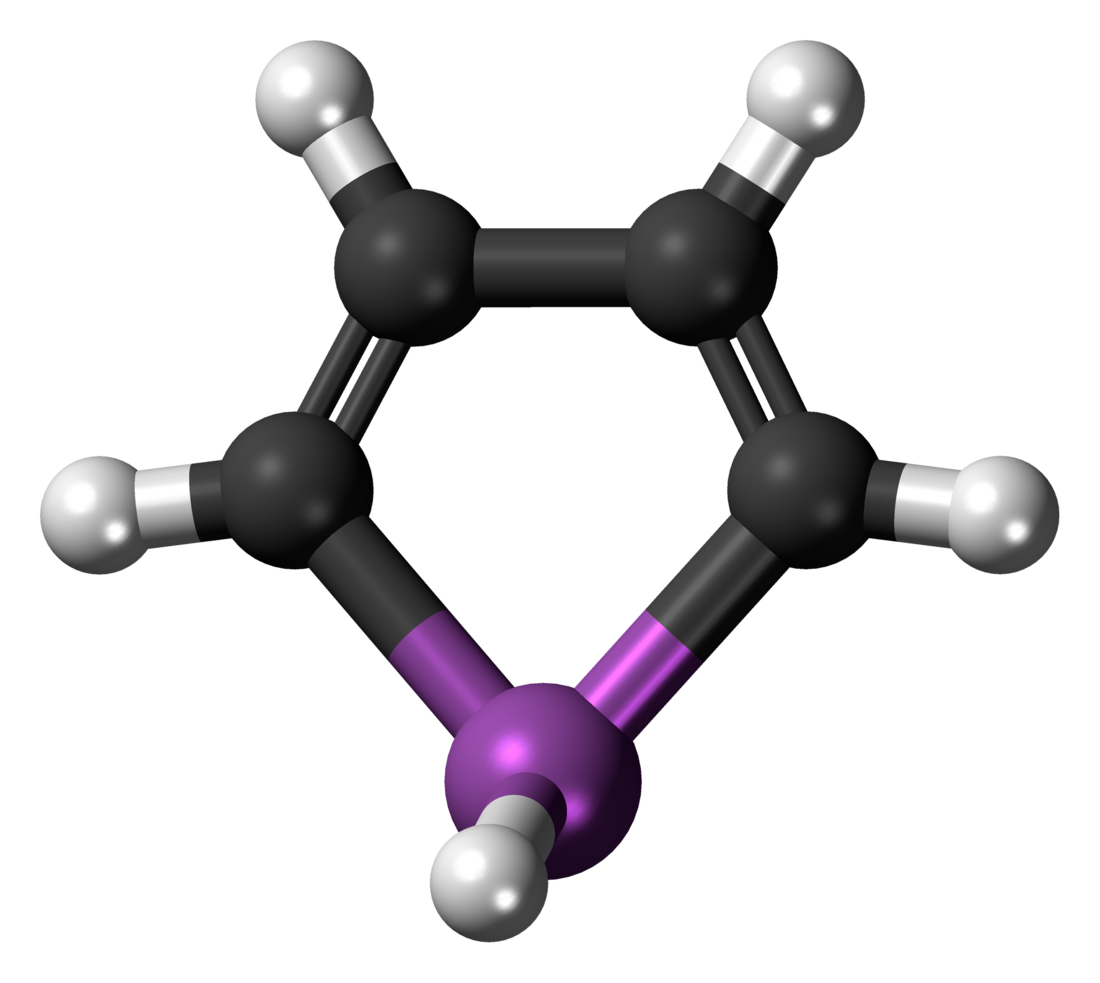
Stibole is a theoretical heterocyclic organic compound, a five-membered ring with the formula C4H4SbH. It is classified as a metallole. It can be viewed as a structural analog of pyrrole, with antimony replacing the nitrogen atom of pyrrole. Stibole itself is very rare, but many substituted derivatives have been synthesized. They are called stiboles.
Remove ads
Reactions
Pentaphenylstibile is prepared from 1,4-dilithio-1,2,3,4-tetraphenylbutadiene and phenylantimony dichloride by a salt metathesis reaction:[1]
- LiCPh=CPh−CPh=CPhLi + PhSbCl2 → Ph4C4SbPh + 2 LiCl (Ph = C6H5)
2,5-Dimethyl-1-phenyl-1H-stibole, for example, can be formed by the reaction of 1,1-dibutyl-2,5-dimethylstannole and dichlorophenylstibine.[2] Stiboles can be used to form ferrocene-like sandwich compounds.[3]
Remove ads
See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads