Top Qs
Timeline
Chat
Perspective
Strontium selenide
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Strontium selenide is an inorganic compound with the chemical formula SrSe.
Remove ads
Preparation
Strontium selenide can be prepared by reducing strontium selenate with hydrogen at 600 °C.[2] It can also be produced by reacting strontium and hydrogen selenide in liquid ammonia.[3]
Properties
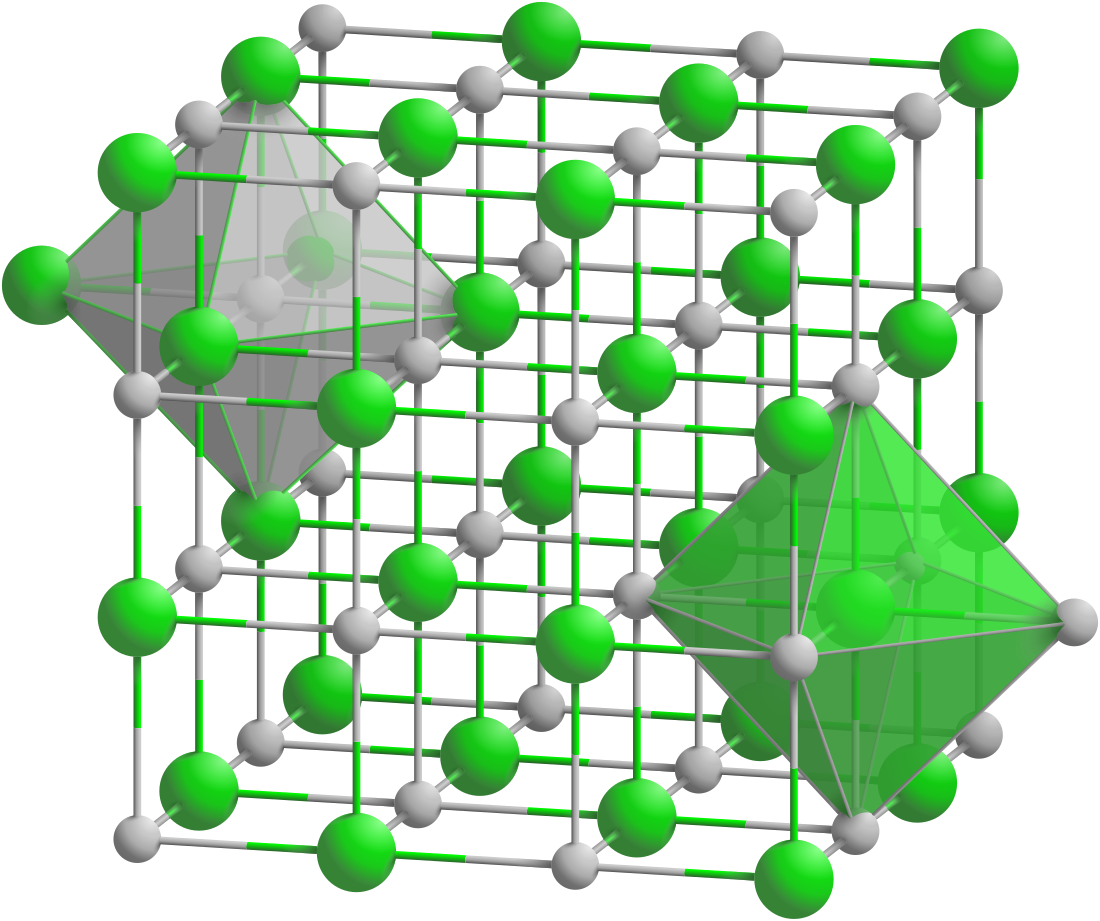
Strontium selenide crystallizes in the orthorhombic crystal system with space group Fm3m. It has a NaCl structure.[4][5] It transforms into a CsCl structure with a space group Pm3m under high pressure (14 GPa).[6]
It reacts with mercury selenide and germanium diselenide at high temperature to obtain the SrHgGeSe4 crystal.[7] It reacts with thorium and selenium at high temperature in the presence of tin to obtain SrTh2Se5.[8]
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads