Top Qs
Timeline
Chat
Perspective
Sulfinylamine
Type of organosulfur compound From Wikipedia, the free encyclopedia
Remove ads
Sulfinylamines (formerly N-sulfinyl amines) are organosulfur compounds with the formula RNSO where R = an organic substituent. These compounds are, formally speaking, derivatives of HN=S=O, i.e. analogues of sulfur dioxide and of sulfur diimide. A common example is N-sulfinylaniline. Sulfinyl amines are dienophile.[1] They undergo [2+2] cycloaddition to ketenes.[2]

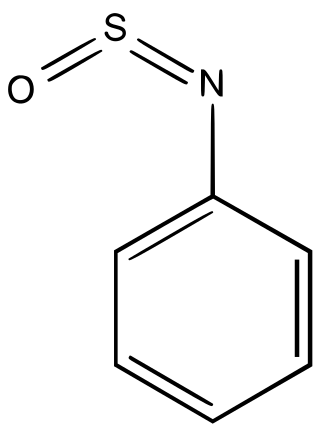
According to X-ray crystallography, sulfinylamines have planar C-N=S=O cores with syn geometry.[3]
Remove ads
Preparation
Sulfinylamines can be made when thionyl chloride SOCl2 reacts with a primary amine.[4] Indeed, the parent thionylamide, HNSO, can be made that way at low temperature.[5]
Reactions
A frustrated Lewis pair, such as tris(tert-butyl) phosphine and tris(pentafluorophenyl)borane, can attach to the NSO chain to yield a R'3P=N+(R)SOB−R"3 compound.[4]
Compounds
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads
