Top Qs
Timeline
Chat
Perspective
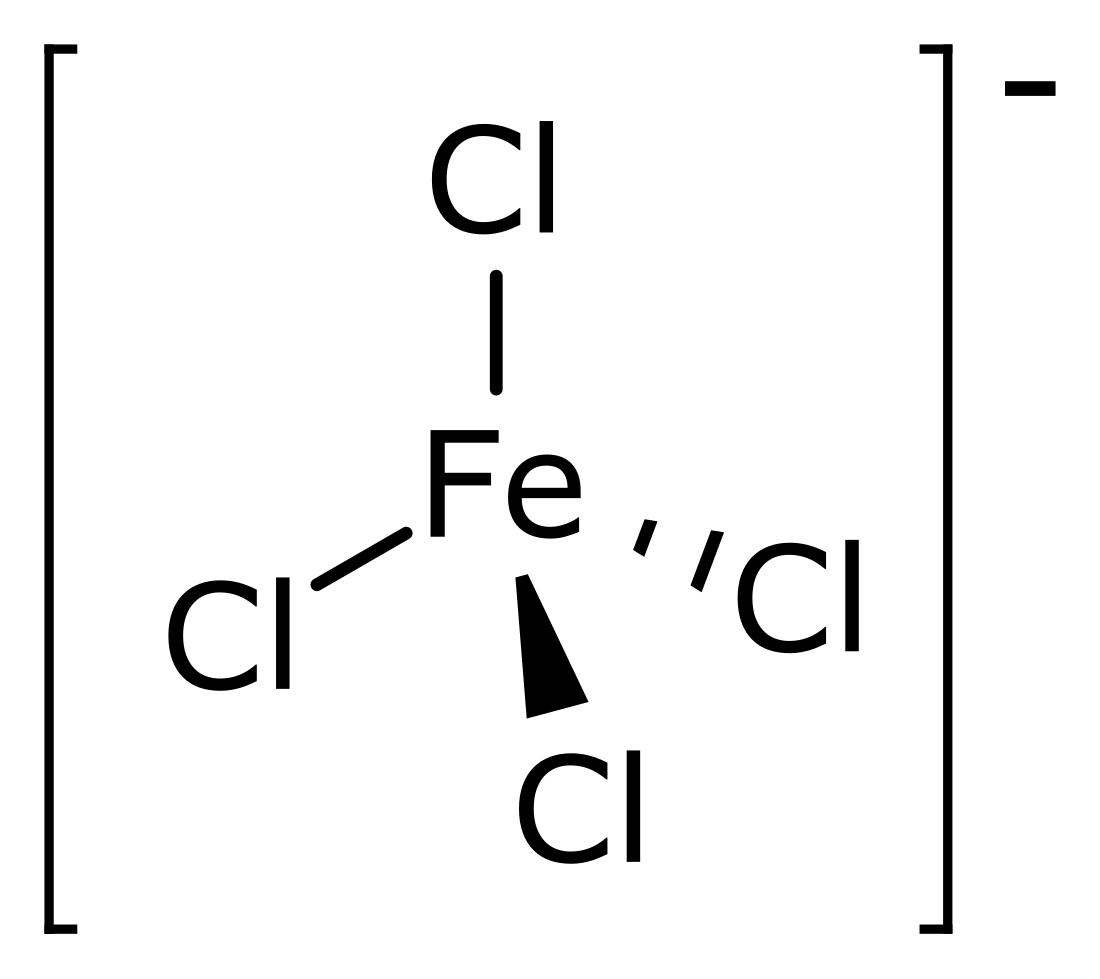
Tetrachloroferrate
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Tetrachloroferrate is the polyatomic ion having chemical formula FeCl−4. The metallate can be formed when ferric chloride (FeCl3) abstracts a chloride ion from various other chloride salts.[1] The resulting tetrachloroferrate salts are typically soluble in non-polar solvents. The tetrachloroferrate anion, with iron(III) in the center, has tetrahedral geometry.[2] It is useful as a non-coordinating anion comparable to perchlorate.[3] Several organoammonium salts have been studied for their novel material properties. 1-Butyl-3-methylimidazolium tetrachloroferrate is one of several ionic liquids that are magnetic.[4] Trimethylchloromethylammonium tetrachloroferrate is a plastic crystal that can behave as a molecular switch in response to several different types of inputs.[5]
Remove ads
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads