Top Qs
Timeline
Chat
Perspective
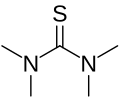
Tetramethylthiourea
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Tetramethylthiourea is an organosulfur compound with the formula ((CH3)2N)2C=S. This commercially available compound is used as a ligand in homogeneous catalysis and in organic synthesis.
Remove ads
Structure
The core of the compound is thiourea, with each nitrogen connected to two methyl groups. The molecule is planar. The C=S bond is 0.02 Å shorter than in thiourea itself.[1]
Reactions
Sulfur is the basic site in tetramethylthiourea. Alkylation occurs at S, affording isothiouronium salts.[2]
Tetramethylthiourea forms many coordination complexes. Two examples tetrahedral CoCl2L2 and linear AuBrL, where L = ((CH3)2N)2C=S.[3][4]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads