Top Qs
Timeline
Chat
Perspective
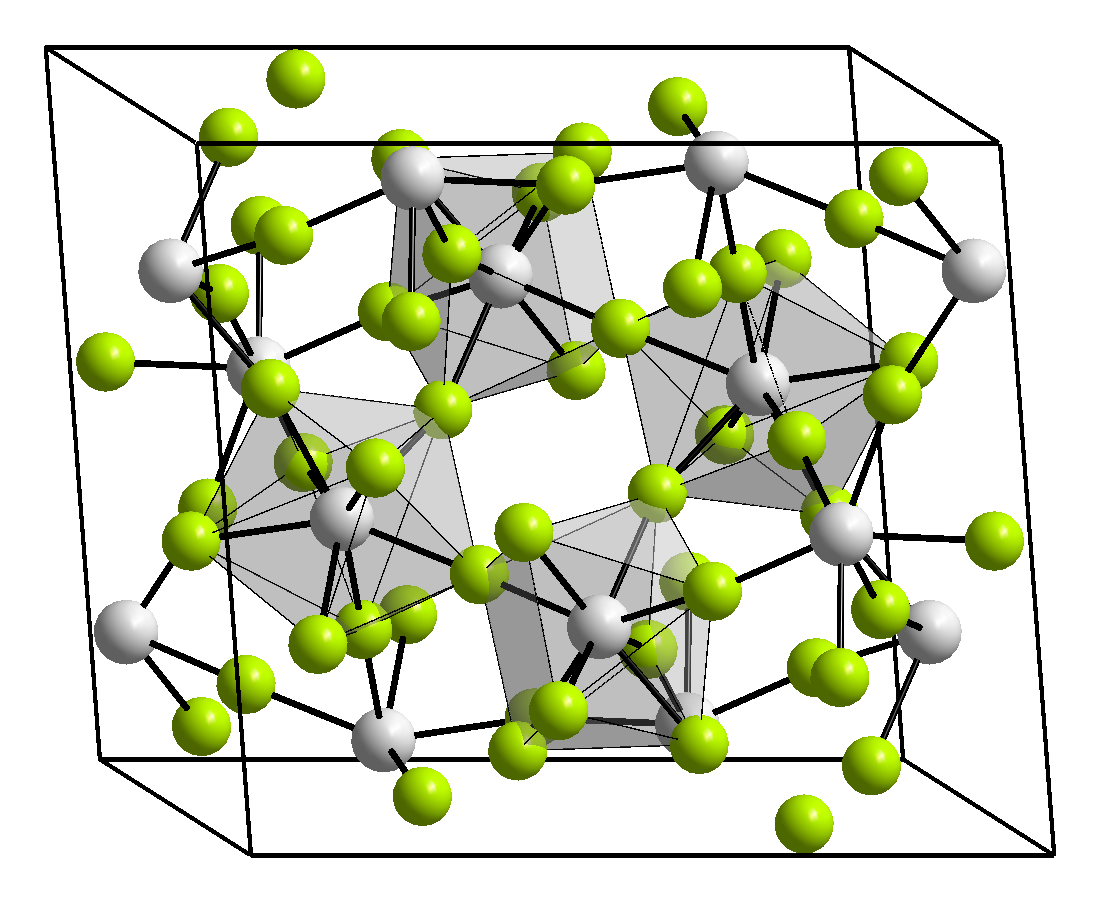
Thorium(IV) fluoride
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Thorium(IV) fluoride (ThF4) is an inorganic chemical compound. It is a white hygroscopic powder which can be produced by reacting thorium with fluorine gas. At temperatures above 500 °C, it reacts with atmospheric moisture to produce ThOF2.[1]
Remove ads
Uses
Despite its (mild) radioactivity, thorium fluoride is used as an antireflection material in multilayered optical coatings. It has excellent optical transparency in the range 0.35–12 μm, and its radiation is primarily due to alpha particles, which can be easily stopped by a thin cover layer of another material.[2][3] However, like all alpha emitters, thorium is potentially hazardous if incorporated, which means safety should focus on reducing or eliminating this danger. In addition to its radioactivity, thorium is also a chemically toxic heavy metal.
Thorium fluoride was used[when?] in making carbon arc lamps, which provided high-intensity illumination for movie projectors and search lights.[4][5]
Remove ads
See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads