Top Qs
Timeline
Chat
Perspective
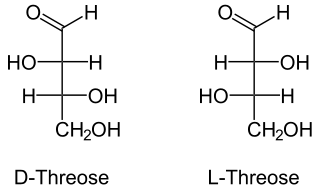
Threose
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Threose is a four-carbon monosaccharide with molecular formula C4H8O4. It has a terminal aldehyde group, rather than a ketone, in its linear chain and so is considered part of the aldose family of monosaccharides. The threose name can be used to refer to both the d- and l-stereoisomers and more generally to the racemic mixture (d/L-, equal parts D- and L-) as well as to the more generic threose structure (absolute stereochemistry unspecified).
The prefix "threo-" which derives from threose (and "erythro-" from a corresponding diastereomer erythrose) offer a useful way to describe general organic structures with adjacent chiral centers, where "the prefixes... designate the relative configuration of the centers".[3] As is depicted in a Fischer projection of d-threose, the adjacent substituents will have a syn orientation in the isomer referred to as "threo", and are anti in the isomer referred to as "erythro".[3][4]
Although often inconsequential, threose in aqueous solution mainly exists as the hydrate owing to the following equilibrium:[5]
- HOCH2CH(OH)CH(OH)CHO + H2O ⇌ HOCH2CH(OH)CH(OH)CH(OH)2
Remove ads
See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads


