Top Qs
Timeline
Chat
Perspective
Tin(IV) sulfide
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Tin(IV) sulfide is a compound with the formula SnS2. A brown, water-insoluble solid, it is a semiconductor with band gap 2.2 eV.[5] It occurs naturally as the rare mineral berndtite.[6]
Remove ads
Synthesis and structure
The compound precipitates as a brown solid upon the addition of H2S to solutions containing tin(IV) species. This reaction is reversed at low pH. It can also be prepared by heating finely ground Sn with excess sulfur.[7]
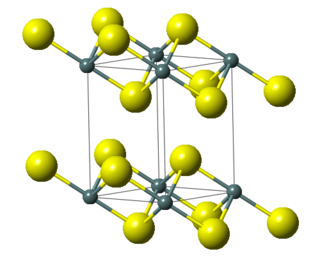
The compound crystallizes in the cadmium iodide motif, with the Sn(IV) situated in "octahedral holes' defined by six sulfide centers.[8]
The material reacts with sulfide salts to give a series of thiostannates with the formula [SnS
2]
m[S]2n−
n.[9] A simplified equation for this depolymerization reaction is:
- SnS2 + S2− → 1/x[SnS32−]x
Remove ads
Potential uses
Crystalline SnS2 has a bronze color and is used in decorative coating[10] where it is known as mosaic gold.
Tin (IV) sulfide has various uses in electrochemistry. It serves as an anode in prototypes of lithium-ion batteries.[11] Intercalation with organometallic reagents is reversible.[12]
It has also been evaluated as a component of supercapacitors, which could be used for energy storage.[13]
Remove ads
See also
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads