Top Qs
Timeline
Chat
Perspective
Titanium tetrafluoride
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
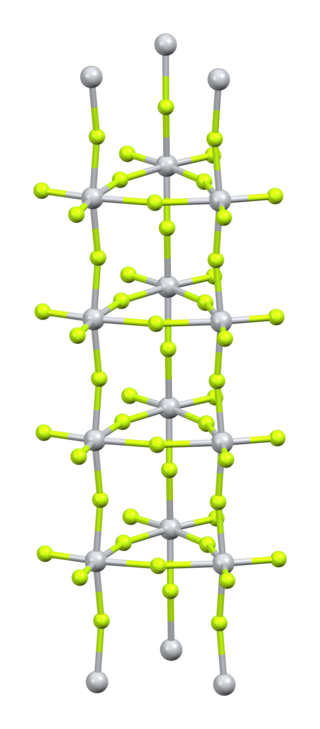
Titanium(IV) fluoride is the inorganic compound with the formula TiF4. It is a white hygroscopic solid. In contrast to the other tetrahalides of titanium, it adopts a polymeric structure.[2] In common with the other tetrahalides, TiF4 is a strong Lewis acid.
Remove ads
Preparation and structure
The traditional method involves treatment of titanium tetrachloride with excess hydrogen fluoride:[3]
- TiCl4 + 4 HF → TiF4 + 4 HCl
Purification is by sublimation, which involves reversible cracking of the polymeric structure.[4] X-ray crystallography reveals that the Ti centres are octahedral, but conjoined in an unusual columnar structure.[5]
Reactions

TiF4 forms adducts with many ligands. One example is the complex cis-TiF4(CH3CN)2, which is formed by treatment with acetonitrile.[6] It is also used as a reagent in the preparation of organofluorine compounds.[7] With fluoride, the cluster [Ti4F18]2- forms. It has an adamantane-like Ti4F6 core.[8]
Related to its Lewis acidity, TiF4 forms a variety of hexafluorides also called hexafluorotitanates. Hexafluorotitanic acid has been used commercially to clean metal surfaces. These salts are stable at pH<4 in the presence of hydrogen fluoride, otherwise they hydrolyze to give oxides.[3]
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads