Top Qs
Timeline
Chat
Perspective
Transition metal complexes of phosphine oxides
Class of chemical compounds From Wikipedia, the free encyclopedia
Remove ads
Transition metal complexes of phosphine oxides are coordination complex containing one or more phosphine oxide ligands. Many phosphine oxides exist and most behave as hard Lewis bases. Almost invariably, phosphine oxides bind metals by formation of M-O bonds.[1]
Remove ads
Structure

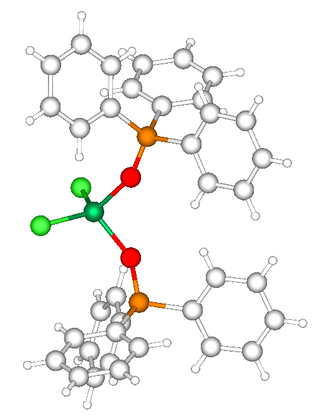
The structure of the phosphine oxide is not strongly perturbed by coordination. The geometry at phosphorus remains tetrahedral. The P-O distance elongates by ca. 2%. In triphenylphosphine oxide, the P-O distance is 1.48 Å.[3] In NiCl2[OP(C6H5)3]2, the distance is 1.51 Å (see figure). A similar elongation of the P-O bond is seen in cis-WCl4(OPPh3)2.[4] The trend is consistent with the stabilization of the ionic resonance structure upon complexation.
Remove ads
Examples
Typically, complexes are derived from hard metal centers. Examples include cis-WCl4(OPPh3)2[4] and NbOCl3(OPPh3)2[5] Trialkylphosphine oxides are more basic (better ligands) than triarylphosphine oxides. One such complex is FeCl2(OPMe3)2 (Me = CH3).[6]
Synthesis and reactions
Most complexes of phosphine oxides are prepared by treatment of a labile metal complex with preformed phosphine oxide. In some cases, the phosphine oxide is unintentionally generated by air-oxidation of the parent phosphine ligand.
Since phosphine oxides are weak Lewis bases, they are readily displaced from their metal complexes. This behavior has led to investigation of mixed phosphine-phosphine oxide ligands, which exhibit hemilability. Typical phosphine-phosphine oxide ligands are Ph2P(CH2)nP(O)Ph2 (Ph = C6H5) derived from bis(diphenylphosphino)ethane (n = 2) and bis(diphenylphosphino)methane (n = 1).[1]
In one case, coordination of the oxide of dppe to W(0) results in deoxygenation, giving an oxotungsten complex of dppe.[7]
Secondary phosphine oxides as ligands
Secondary phosphine oxides have the formula R2P(O)H.[8] They tautomerize to small amounts of the hydroxy tautomer R2P-OH. Regardless, the hydroxy tautomer forms a wide variety of complexes with transition metals. In contrast to O-bonded phosphine oxide ligands, the P-bonded phosphine oxides are strong field ligands. These ligands, which tend to engage in intramolecular hydrogen bonds. Illustrative is the complex derived from dimethylphosphine oxide, PtH(PMe2OH)2(PMe2O) (Me = CH3).[9]
The pattern also applies to several phosphorus compounds including phosphorous acid, which forms complexes as P(OH)3. The complex platinum pop is one example.
The Kläui ligand is the anion {(C5H5)Co[(CH3O)2PO]3}−. It is derived from the trimethylphosphite ligand by dealkylation. In this case the "ligand" is a complex of cobalt that also binds to other metals in a tridentate manner.[10]
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads

