Top Qs
Timeline
Chat
Perspective
Tributyltin hydride
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
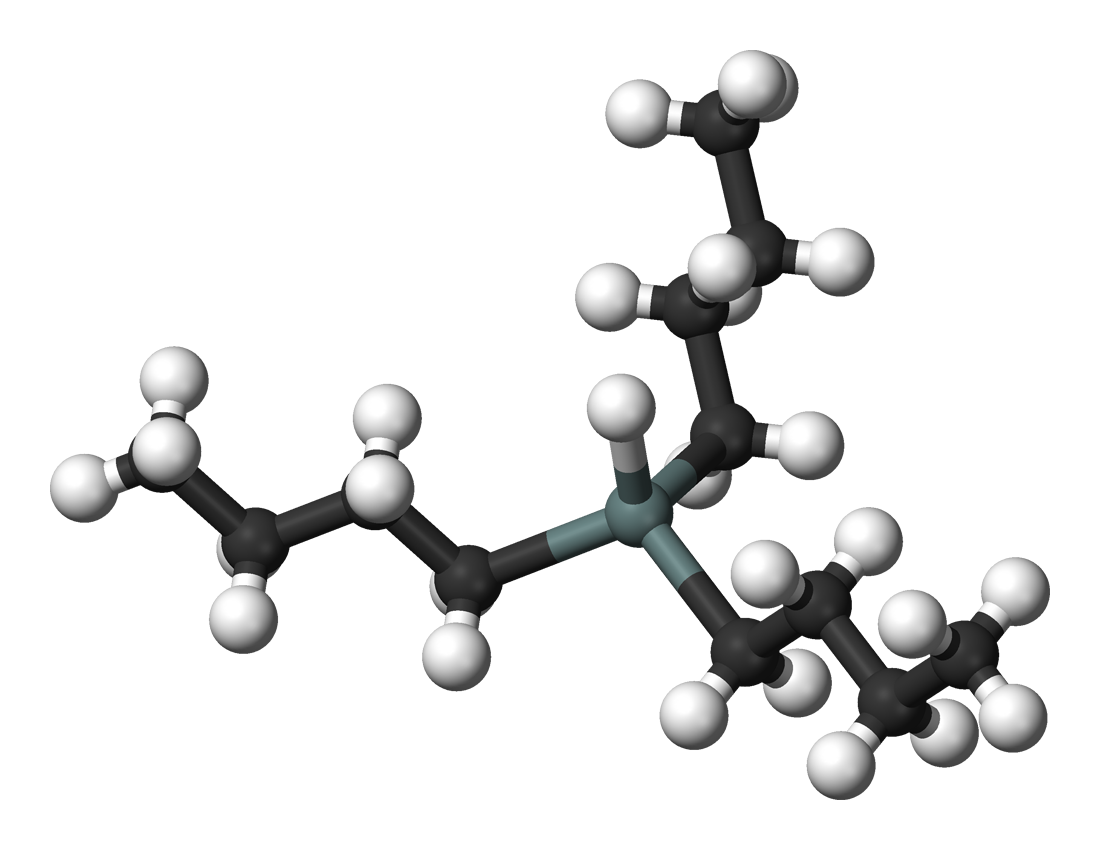
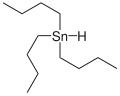
Tributyltin hydride is an organotin compound with the formula (C4H9)3SnH. It is a colorless liquid that is soluble in organic solvents. The compound is used as a source of hydrogen atoms in organic synthesis.
Remove ads
Synthesis and characterization
The compound is produced by reduction of tributyltin oxide with polymethylhydrosiloxane:[2][3]
- 2 "[MeSi(H)O]n" + (Bu3Sn)2O → "[MeSi(OH)O]n" + 2 Bu3SnH
It can also be synthesized by a reduction of tributyltin chloride with lithium aluminium hydride.[4]
The hydride is a distillable liquid that is mildly sensitive to air, decomposing to (Bu3Sn)2O. Its IR spectrum exhibits a strong band at 1814 cm−1 for νSn−H.
Remove ads
Applications
It is a specialized reagent in organic synthesis. Combined with azobisisobutyronitrile (AIBN) or by irradiation with light, tributyltin hydride converts organic halides (and related groups) to the corresponding hydrocarbon. This process occurs via a radical chain mechanism involving the radical Bu3Sn•.[5][6] The radical abstracts a H• from another equivalent of tributyltin hydride, propagating the chain. Tributyltin hydride's utility as a H• donor can be attributed to its relatively weak bond strength (78 kcal/mol).[7]
It is the reagent of choice for hydrostannylation reactions:[8]
- RC2R′ + HSnBu3 → RC(H)=C(SnBu3)R′
Remove ads
See also
References
Further reading
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads