Top Qs
Timeline
Chat
Perspective
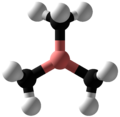
Trimethylborane
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Trimethylborane (TMB) is a toxic, pyrophoric gas with the formula B(CH3)3 (which can also be written as Me3B, with Me representing methyl).
Remove ads
Properties
As a liquid it is colourless. The strongest line in the infrared spectrum is at 1330 cm−1 followed by lines at 3010 cm−1 and 1185 cm−1.
Its melting point is −161.5 °C, and its boiling point is −20.2 °C.
Vapour pressure is given by log P = 6.1385 + 1.75 log T − 1393.3/T − 0.007735 T, where T is temperature in kelvins.[5] Molecular weight is 55.914. The heat of vapourisation is 25.6 kJ/mol.[4]
Remove ads
Preparation
Trimethylborane was first described in 1862 by Edward Frankland,[6] who also mentioned its adduct with ammonia.[7] Due to its dangerous nature the compound was no longer studied until 1921, when Alfred Stock and Friedrich Zeidler took advantage of the reaction between boron trichloride gas and dimethylzinc.[8] Although the substance can be prepared using Grignard reagents the output is contaminated by unwanted products from the solvent. Trimethylborane can be made on a small scale with a 98% yield by reacting trimethylaluminium in hexane with boron tribromide in dibutyl ether as a solvent.[5] Yet other methods are reacting tributyl borate with trimethylaluminium chloride, or potassium tetrafluoroborate with trimethylaluminium,[9] or adding boron trifluoride in ether to methyl magnesium iodide.[10]
Remove ads
Reactions
Summarize
Perspective
Trimethylborane spontaneously ignites in air if the concentration is high enough. It burns with a green flame producing soot.[11] Slower oxidation with oxygen in a solvent or in the gas phase can produce dimethyltrioxadiboralane, which contains a ring of two boron and three oxygen atoms. However the major product is dimethylborylmethylperoxide, which rapidly decomposes to dimethoxymethylborane.[12]
Trimethylborane is a strong Lewis acid. B(CH3)3 can form an adduct with ammonia: (NH3):B(CH3)3.[13] as well as other Lewis bases. The Lewis acid properties of B(CH3)3 have been analyzed by the ECW model yielding EA= 2.90 and CA= 3.60. When trimethylborane forms an adduct with trimethylamine, steric repulsion between the methyl groups on the B and N results. The ECW model can provide a measure of this steric effect.
Trimethylborane reacts with water and chlorine at room temperature. It also reacts with grease but not with teflon or glass.[5]
Trimethylborane reacts with diborane to disproportionate to form methyldiborane and dimethyldiborane: (CH3)BH2.BH3 and (CH3)2BH.BH3.
It reacts as a gas with trimethylphosphine to form a solid Lewis salt with a heat of formation of −41 kcal per mol. This adduct has a heat of sublimation of −24.6 kcal/mol. No reaction occurs with trimethylarsine or trimethylstibine.[10]
Methyl lithium reacting with the Trimethylborane produces a tetramethylborate salt: LiB(CH3)4.[14] The tetramethylborate ion has a negative charge and is isoelectronic with neopentane, tetramethylsilane, and the tetramethylammonium cation.
Use
Trimethylborane has been used as a neutron counter.[15] For this use it has to be very pure.[13] It is also used in chemical vapour deposition where boron and carbon need to be deposited together.
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads