Top Qs
Timeline
Chat
Perspective
Triphenylarsine
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
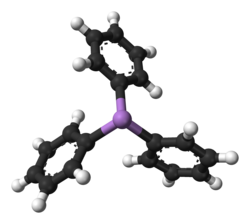
Triphenylarsine is the chemical compound with the formula As(C6H5)3. This organoarsenic compound, often abbreviated AsPh3, is a colorless crystalline solid that is used as a ligand and a reagent in coordination chemistry and organic synthesis. The molecule is pyramidal with As-C distances of 1.942–1.956 Å and C-As-C angles of 99.6–100.5°.[1]
Remove ads
Preparation
Triphenylarsine is prepared by treating phenyl lithium with arsenic tribromide. The method can be used to prepare many analogues with diverse aryl groups.[2]
Triphenylarsine can also be prepared by the Wurtz-like reaction of arsenic trichloride with chlorobenzene using sodium as the reducing agent:[3]
- AsCl3 + 3 PhCl + 6 Na → AsPh3 + 6 NaCl
Reactions
Reaction of triphenylarsine with lithium gives lithium diphenylarsenide and phenyllithium:[4]
- AsPh3 + 2 Li → LiAsPh2 + LiPh
Triphenylarsine is the precursor to tetraphenylarsonium chloride, [AsPh4]Cl, a popular precipitating agent.[3]
Many metal-arsine complexes have been prepared using AsPh3. Examples include IrCl(CO)(AsPh3)2, RhCl(AsPh3)3, and Fe(CO)4(AsPh3).[5]
Treatment of triphenylarsine with hydrogen peroxide gives triphenylarsine oxide: [6]
- AsPh3 + H2O2 → OAsPh3 + H2O
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads