Top Qs
Timeline
Chat
Perspective
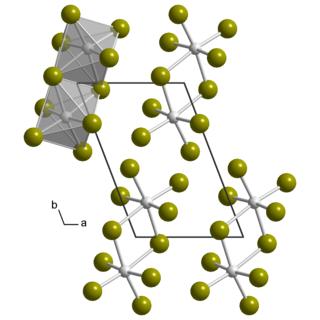
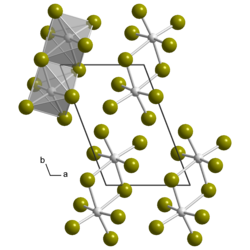
Uranium pentabromide
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Uranium pentabromide is an inorganic chemical compound with the formula U2Br10.
Remove ads
Synthesis
The compound is made by reacting the elements in an acetonitrile solvent, or by reacting bromine with uranium metal or uranium tetrabromide at 55 °C (131 °F; 328 K).[1]
Properties
Uranium pentabromide is a hygroscopic dark brown solid that decomposes in water and most organic solvents, the exceptions being acetonitrile or dichloromethane.[1] The compound is rather unstable and difficult to purify,[2] decomposing at 80 °C (176 °F; 353 K) into uranium tetrabromide and bromine.[3] The crystal structure is the same as that of β-UCl5, which is triclinic and consists of U2Br10 dimers.[4]
Remove ads
Complexes
Stable complexes of the form UBr5L are known with such ligands as triphenylphosphine oxide and hexamethylphosphoramide, and are obtained by brominating UBr4 in the presence of the desired ligand.[2] In addition, it is possible to obtain a hexabromouranate(V) salt by reacting UBr5 with a monovalent bromide in thionyl bromide:[1]
- U2Br10 + 2MBr → 2M+[UBr6]−
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads