Top Qs
Timeline
Chat
Perspective
Vanadium(II) sulfate
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
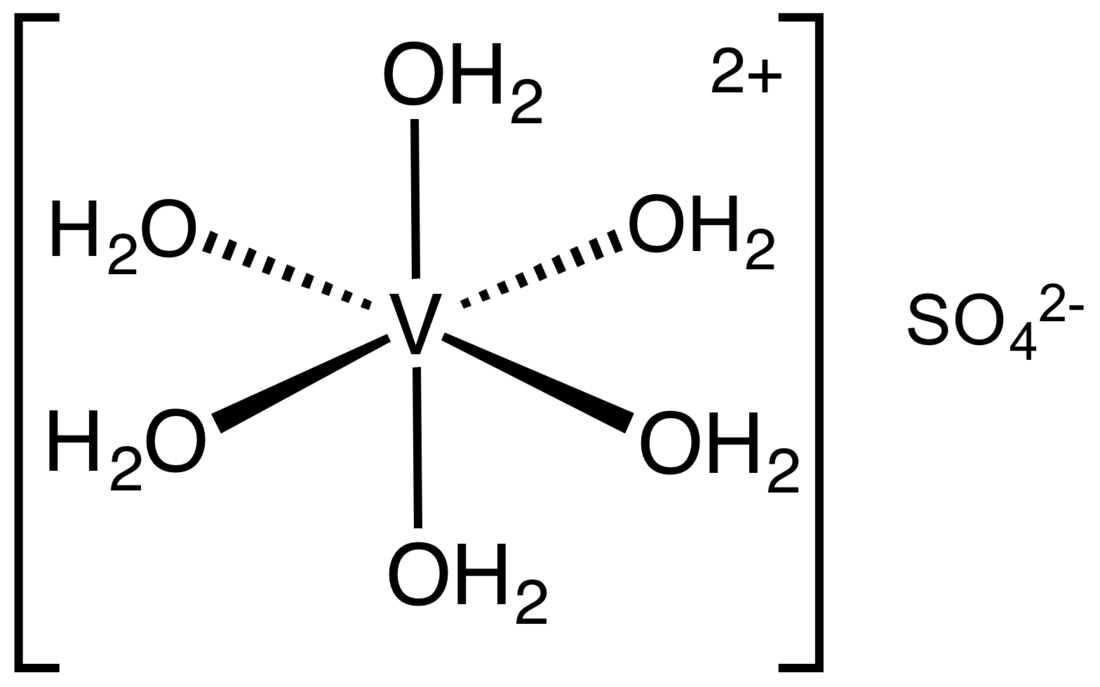
Vanadium(II) sulfate describes a family of inorganic compounds with the formula VSO4(H2O)x where 0 ≤ x ≤ 7. The hexahydrate is most commonly encountered. It is a violet solid that dissolves in water to give air-sensitive solutions of the aquo complex. The salt is isomorphous with [Mg(H2O)6]SO4. Compared to the V–O bond length of 191 pm in [V(H2O)6]3+, the V–O distance is 212 pm in the [V(H2O)6]SO4. This nearly 10% elongation reflects the effect of the lower charge, hence weakened electrostatic attraction.[1]
The heptahydrate has also been crystallized. The compound is prepared by electrolytic reduction of vanadyl sulfate in sulfuric acid.[1][2] The crystals also feature [V(H2O)6]2+ centers but with an extra water of crystallization. The salt is isomorphous with ferrous sulfate heptahydrate.[3] A related salt is vanadous ammonium sulfate, (NH4)2V(SO4)2·6H2O, a Tutton's salt isomorphous with ferrous ammonium sulfate.[4]
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads