Top Qs
Timeline
Chat
Perspective
Ytterbium(II) bromide
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Ytterbium(II) bromide is an inorganic compound with the chemical formula YbBr2.
Remove ads
Preparation
Ytterbium(II) bromide can be produced by the reduction reaction of ytterbium(III) bromide and hydrogen at 500~600 °C:[1]
- 2 YbBr3 + H2 → 2 YbBr2 + 2 HBr
The ammonia compound can be obtained by reacting metallic ytterbium and ammonium bromide in liquid ammonia at −78 °C. The ammonia compound can be decomposed in high vacuum at 200 °C to obtain ytterbium(II) bromide:[1]
- Yb + 2 NH4Br → YbBr_2 + 2 NH3 + H2
Ytterbium(II) bromide can also be prepared by vacuum reduction of metallic ytterbium at 960 °C:[2]
- Yb + 2 YbBr3 → 3 YbBr2
Remove ads
Properties
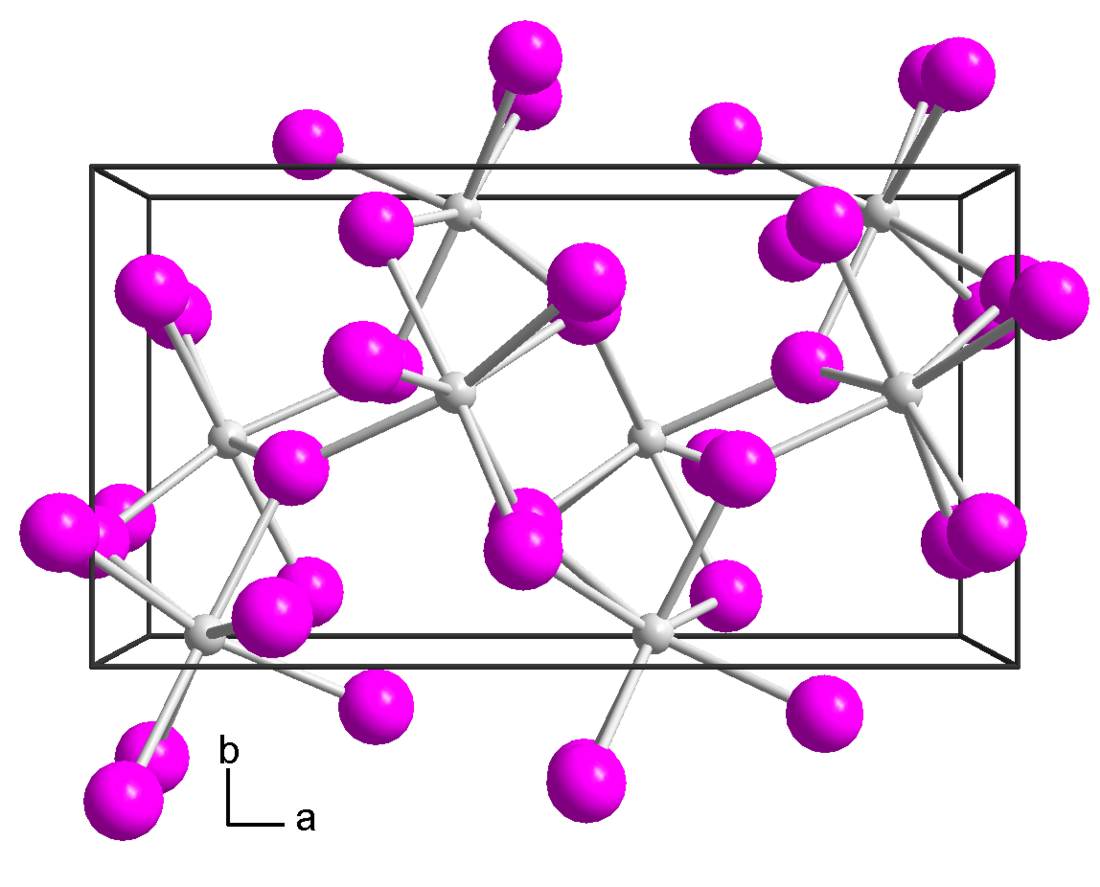
Ytterbium(II) bromide is a light yellow solid and is highly hygroscopic. It can only be stored in an inert atmosphere or high vacuum. It is unstable in air or moisture and rapidly converts to the oxybromide and releases hydrogen gas. Ytterbium(II) bromide belongs to the orthorhombic crystal system, with SrI2 structure and space group Pbca,[3] or CaCl2 structure and space group Pnnm. Its unit cell parameters are a=6.63 Å, b=6.93 Å, c=4.47Å.[1][3]
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads