Top Qs
Timeline
Chat
Perspective
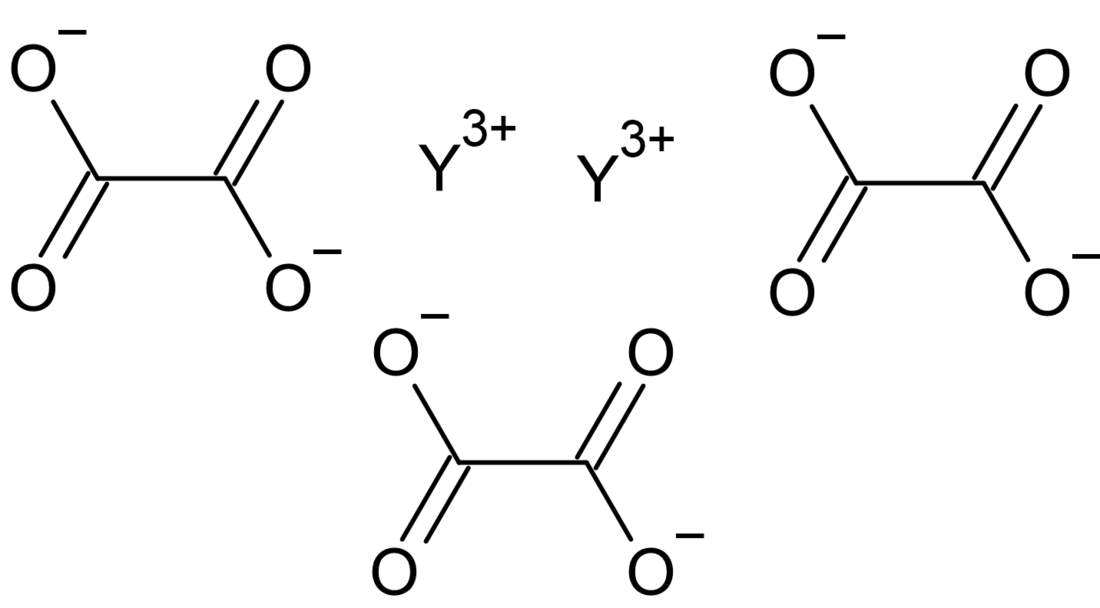
Yttrium oxalate
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Yttrium oxalate is an inorganic compound, a salt of yttrium and oxalic acid with the chemical formula Y2(C2O4)3.[3] The compound does not dissolve in water and forms crystalline hydrates—colorless crystals.[4]
Remove ads
Synthesis
Summarize
Perspective
Precipitation of soluble yttrium salts with oxalic acid:[5]
Remove ads
Properties
Summarize
Perspective
Yttrium oxalate is highly insoluble in water and converts to the oxide when heated.[6] Yttrium oxalate forms crystalline hydrates (colorless crystals) with the formula Y2(C2O4)3•n H2O, where n = 4, 9, and 10.
Decomposes when heated:
The solubility product of yttrium oxalate at 25 °C is 5.1 × 10−30.[1]
The trihydrate Y2(C2O4)3•3H2O is formed by heating more hydrated varieties at 110 °C.[7]
Y2(C2O4)3•2H2O, which is formed by heating the decahydrate at 210 °C) forms monoclinic crystals with unit cell dimensions a=9.3811 Å, b=11.638 Å, c=5.9726 Å, β=96.079°.[8]
Remove ads
Related
Summarize
Perspective
Several yttrium oxalate double salts are known containing additional cations. Also a mixed-anion compound with carbonate is known.
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads