Top Qs
Timeline
Chat
Perspective
Zinc carbonate
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Zinc carbonate is the inorganic compound with the formula ZnCO3. It is a white solid that is insoluble in water. It exists in nature as the mineral smithsonite. It is prepared by treating cold solutions of zinc sulfate with potassium bicarbonate. Upon warming, it converts to basic zinc carbonate (Zn5(CO3)2(OH)6).[6]
Remove ads
Structure
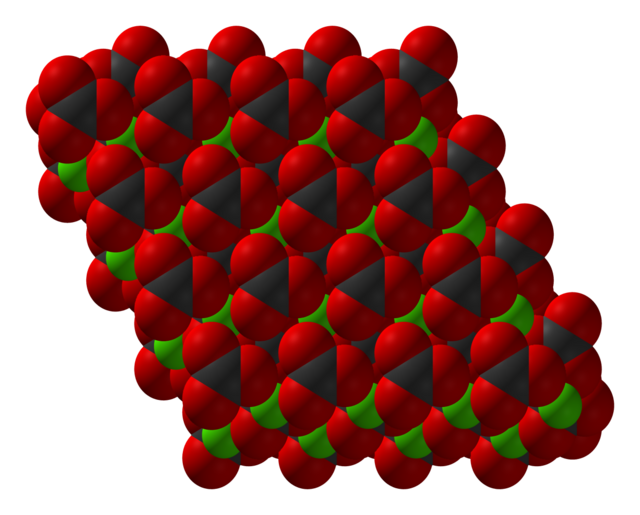
Zinc carbonate adopts the same structure as calcite (calcium carbonate).[7] Zinc is octahedral and each carbonate is bonded to six Zn centers such that oxygen atoms are three-coordinate.
References
Cited sources
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads
