Dinitrogen tetroxide
chemical compound From Wikipedia, the free encyclopedia
Remove ads
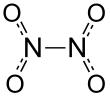
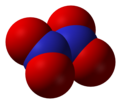
Dinitrogen tetroxide, also known as nitrogen tetroxide or dinitrogen tetraoxide, is a chemical compound. Its chemical formula is N2O4. It contains nitrogen in its +4 oxidation state. It contains nitrogen and oxide ions.
Remove ads
Properties
It is a colorless gas, although it is sometimes polluted with nitrogen dioxide. It is very corrosive and a strong oxidizing agent. It can ignite on contact with hydrazine. It can be made by bonding two nitrogen dioxide molecules together at a low temperature or a high pressure.
Preparation
It is made by bonding nitrogen dioxide molecules together in pairs.
Uses
It is used as a rocket propellant, along with hydrazine. This mixture is good since it does not have to be ignited. It is used similar to nitrogen dioxide to make nitric acid. It can react with metals to make nitrates.
Safety
Dinitrogen is highly toxic and corrosive. Some astronauts breathed it and had to go to a hospital.
Related pages
Sources
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads