Hydrogen ion
Wikimedia disambiguation page From Wikipedia, the free encyclopedia
Remove ads
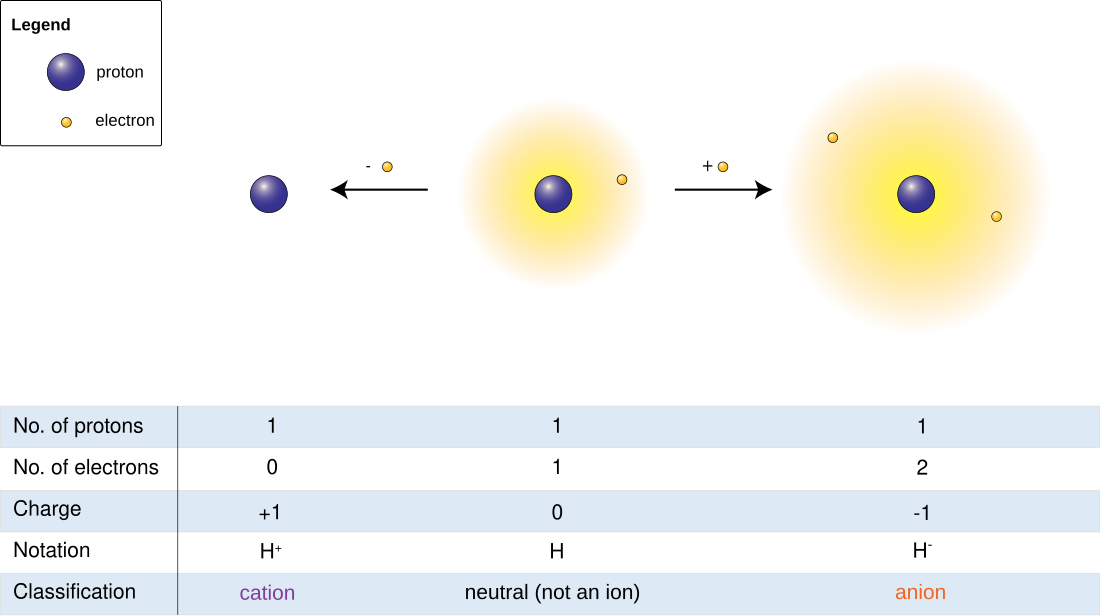
Hydrogen ion is recommended by IUPAC as a general term for all ions of hydrogen and its isotopes.[1] Depending on the charge of the ion, there are two types: positively charged ions and negatively charged ions.

Cation (positively charged)
When hydrogen loses its electron, the cations can be formed:
- Hydron: general name referring to the positive ion of any hydrogen isotope (H+)
- Proton: 1H+ (i.e. the cation of protium)
- Deuteron: 2H+, D+
- Triton: 3H+, T+
The ions produced by the reaction of these cations with water, as well as their hydrates, are also called 'hydrogen ions'.
In connection with acids, "hydrogen ions" usually refers to hydrons.
Remove ads
Anion (negatively charged)
Hydrogen anions are formed when additional electrons are acquired:
- Hydride: general name referring to the negative ion of any hydrogen isotope (H−)
- Protide: 1H−
- Deuteride: 2H−, D −
- Tritide: 3H−, T −
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads