Rutherford model
model of the atom devised by Ernest Rutherford From Wikipedia, the free encyclopedia
Remove ads
The Rutherford model is a model by Ernest Rutherford.[1] It was created with the help of a experiment that used gold foil to direct alpha (∝)particles.[2][3] Thomson's plum pudding model was discovered to be wrong due to this new theory.[4] However, Rutherford's model also had mistakes, so the Bohr model came to replace his model.[5]
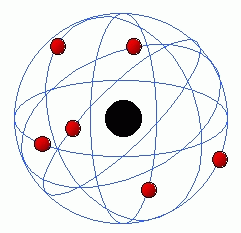
Remove ads
Bohr model
The Bohr model is an improved version of the Rutherford model. It said that electrons have different energy levels, but the original Rutherford model didn't say that, and instead said the model orbited like planets around the sun.[6]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads
