
核碱基
维基百科,自由的 encyclopedia
核碱基(英语:nucleobase)又称含氮碱基(nitrogenous base)[1][2],生物学常简称碱基(base),是一类性质为碱性且拥有氮的化合物,也是在DNA和RNA中起配对作用的部分。例如嘧啶类是核碱基,胺类是最典型的含氮碱基;核碱基与含氮碱基两者称呼只是强调重点不同而已,前者以生物学的角度强调此碱在核苷酸中[3],后者以化学的角度强调此有机碱含有氮原子。
核碱基都是杂环化合物,其氮原子位于环上或取代氨基上,其中一部分(取代氨基,以及嘌呤环的1位氮、嘧啶环的3位氮)直接参与碱基配对。
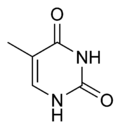
常见的核碱基共有5种:胞嘧啶(缩写C)、鸟嘌呤(G)、腺嘌呤(A)、胸腺嘧啶(T,通常为DNA专有)和尿嘧啶(U,通常为RNA专有)。腺嘌呤和鸟嘌呤属于嘌呤族(缩写作R),它们具有双环结构。胞嘧啶、尿嘧啶、胸腺嘧啶属于嘧啶族(Y),它们的环系是一个六元杂环。RNA中,尿嘧啶取代了胸腺嘧啶的位置。胸腺嘧啶比尿嘧啶多一个5位甲基,这个甲基增大了遗传的准确性。
| 鸟嘌呤(G) | 腺嘌呤(A) | |

|

| |

|

| |
| 胞嘧啶(C) | 胸腺嘧啶(T) | 尿嘧啶(U) |

|
|

|