Top Qs
Timeline
Chat
Perspective
Beryllium hydride
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Beryllium hydride (systematically named poly[beryllane(2)] and beryllium dihydride) is an inorganic compound with the chemical formula (BeH
2)n (also written ([BeH
2])n or BeH
2). This alkaline earth hydride is a colourless solid that is insoluble in solvents that do not decompose it. Unlike the ionically bonded hydrides of the heavier Group 2 elements, beryllium hydride is covalently bonded[1] (three-center two-electron bond).
Remove ads
Synthesis
Unlike the other group 2 metals, beryllium does not react with hydrogen.[3] Instead, BeH2 is prepared from preformed beryllium(II) compounds. It was first synthesized in 1951 by treating dimethylberyllium, Be(CH3)2, with lithium aluminium hydride, LiAlH4.[4]
Purer BeH2 forms from the pyrolysis of di-tert-butylberyllium, Be(C[CH3]3)2 at 210°C.[5]
A route to highly pure samples involves the reaction of triphenylphosphine, PPh3, with beryllium borohydride, Be(BH4)2:[1]
- Be(BH4)2 + 2 PPh3 → BeH2 + 2 Ph3PBH3
Remove ads
Structure
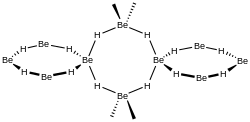
Gaseous form

Isolated molecules of BeH
2 (sometimes called dihydridoberyllium and written [BeH
2] to emphasize the differences with the solid state) are only stable as a dilute gas. When condensed, unsolvated BeH
2 will spontaneously autopolymerise.
Free molecular BeH2 produced by high-temperature electrical discharge has been confirmed to have linear geometry with a Be-H bond length of 133.376 pm. Its hybridization is sp.[6]
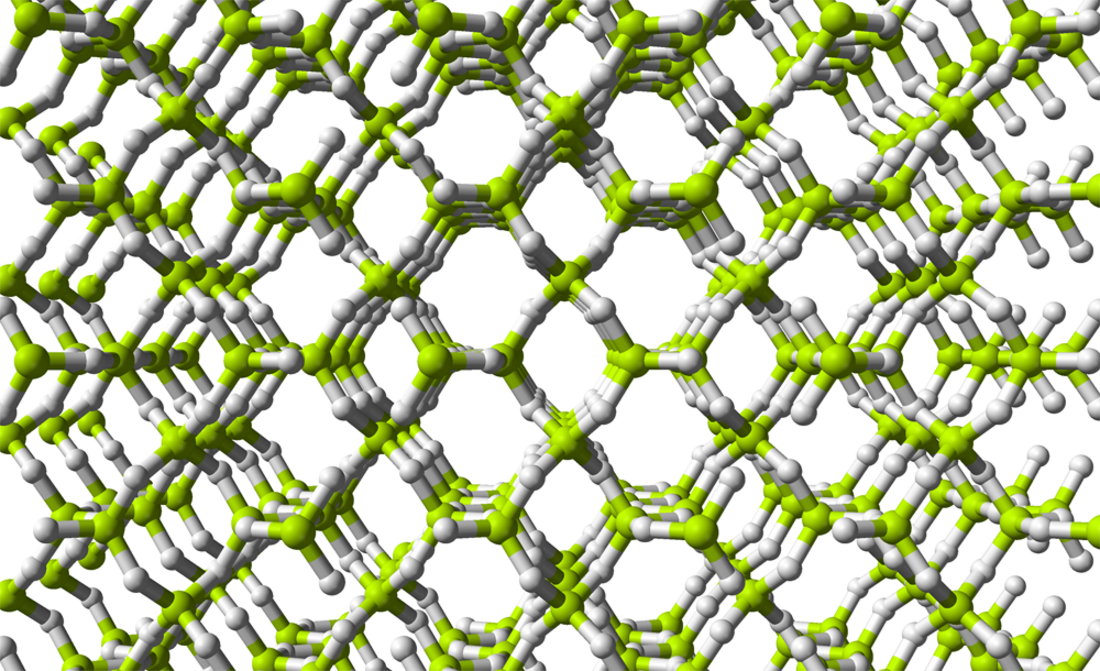
Condensed Beryllium hydride
BeH2 is usually formed as an amorphous white solid, but a hexagonal crystalline form with a higher density (~0.78 g/cm3) was reported,[7] prepared by heating amorphous BeH2 under pressure, with 0.5-2.5% LiH as a catalyst.
A more recent investigation found that crystalline beryllium hydride has a body-centred orthorhombic unit cell, containing a network of corner-sharing BeH4 tetrahedra, in contrast to the flat, hydrogen-bridged, infinite chains previously thought to exist in crystalline BeH2.[8]
Studies of the amorphous form also find that it consists of a network of corner shared tetrahedra.[9]
Remove ads
Chemical properties
Reaction with water and acids
Beryllium hydride reacts slowly with water but is rapidly hydrolysed by acid such as hydrogen chloride to form beryllium chloride.[3]
- BeH2 + 2 H2O → Be(OH)2 + 2 H2
- BeH2 + 2 HCl → BeCl2 + 2 H2
Reaction with Lewis bases
The two-coordinate hydridoberyllium group can accept an electron-pair donating ligand (L) into the molecule by adduction:[10]
- [BeH
2] + L → [BeH
2L]
Because these reactions are energetically favored, beryllium hydride has Lewis-acidic character.
The reaction with lithium hydride (in which the hydride ion is the Lewis base), forms sequentially LiBeH3 and Li2BeH4.[3] The latter contains the tetrahydridoberyllate(2-) anion BeH2−
4.
Beryllium hydride reacts with trimethylamine, N(CH3)3 to form a dimeric adduct with bridging hydrides.[11] However, with dimethylamine, HN(CH3)2 it forms a trimeric beryllium diamide, [Be(N(CH3)2)2]3, and hydrogen.[3]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads