Top Qs
Timeline
Chat
Perspective
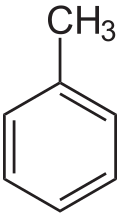
Toluene
Aromatic hydrocarbon From Wikipedia, the free encyclopedia
Remove ads
Toluene (/ˈtɒl.juiːn/), also known as toluol (/ˈtɒl.ju.ɒl, -ɔːl, -oʊl/), is a substituted aromatic hydrocarbon[15] with the chemical formula C6H5CH3, often abbreviated as PhCH3, where Ph stands for the phenyl group. It is a colorless, water-insoluble liquid with the odor associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) attached to a phenyl group by a single bond. As such, its systematic IUPAC name is methylbenzene. Toluene is predominantly used as an industrial feedstock and a solvent.
As the solvent in some types of paint thinner, permanent markers, contact cement and certain types of glue, toluene is sometimes used as a recreational inhalant[16] and has the potential of causing severe neurological harm.[17][18]
Remove ads
History
The compound was first isolated in 1837 through a distillation of pine oil by Pierre Joseph Pelletier and Filip Neriusz Walter, who named it rétinnaphte.[19][20] In 1841, Henri Étienne Sainte-Claire Deville isolated a hydrocarbon from balsam of Tolu (an aromatic extract from the tropical Colombian tree Myroxylon balsamum), which Deville recognized as similar to Walter's rétinnaphte and to benzene; hence he called the new hydrocarbon benzoène.[21][22][23] In 1843, Jöns Jacob Berzelius recommended the name toluin.[24] In 1850, French chemist Auguste Cahours isolated from a distillate of wood a hydrocarbon which he recognized as similar to Deville's benzoène and which Cahours named toluène.[25][26]
Remove ads
Chemical properties
Summarize
Perspective
The distance between carbon atoms in the toluene ring is 0.1399 nm. The C-CH3 bond is longer at 0.1524 nm, while the average C-H bond length is 0.111 nm.[27]
Ring reactions
Toluene reacts as a normal aromatic hydrocarbon in electrophilic aromatic substitution.[28][29][30] Because the methyl group has greater electron-releasing properties than a hydrogen atom in the same position, toluene is more reactive than benzene toward electrophiles. It undergoes sulfonation to give p-toluenesulfonic acid, and chlorination by Cl2 in the presence of FeCl3 to give ortho- and para- isomers of chlorotoluene.
Nitration of toluene gives mono-, di-, and trinitrotoluene, all of which are widely used. Dinitrotoluene is the precursor to toluene diisocyanate, a precursor to polyurethane foam. Trinitrotoluene (TNT) is an explosive.
Complete hydrogenation of toluene gives methylcyclohexane. The reaction requires a high pressure of hydrogen and a catalyst.
Side chain reactions
The C-H bonds of the methyl group in toluene are benzylic, therefore they are weaker than C-H bonds in simpler alkanes. Reflecting this weakness, the methyl group in toluene undergoes a variety of free radical reactions. For example, when heated with N-bromosuccinimide (NBS) in the presence of AIBN, toluene converts to benzyl bromide. The same conversion can be effected with elemental bromine in the presence of UV light or even sunlight.
Toluene may also be brominated by treating it with HBr and H2O2 in the presence of light.[31]
- C6H5CH3 + Br2 → C6H5CH2Br + HBr
Benzoic acid and benzaldehyde are produced commercially by partial oxidation of toluene with oxygen. Typical catalysts include cobalt or manganese naphthenates.[32] Related but laboratory-scale oxidations involve the use of potassium permanganate to yield benzoic acid and chromyl chloride to yield benzaldehyde (Étard reaction).
The methyl group in toluene undergoes deprotonation only with very strong bases; its pKa is estimated using acidity trends to be approximately 43 in dimethyl sulfoxide (DMSO)[33][34] and its ion pair acidity is extrapolated to be 41.2 in cyclohexylamine (Cesium Cyclohexylamide) using a Bronsted correlation.[35][36]
Miscibility
Toluene is miscible (soluble in all proportions) with ethanol, benzene, diethyl ether, acetone, chloroform, glacial acetic acid and carbon disulfide, but immiscible with water.[37]
Remove ads
Production
Summarize
Perspective
Toluene occurs naturally at low levels in crude oil and is a byproduct in the production of gasoline by a catalytic reformer or ethylene cracker. It is also a byproduct of the production of coke from coal. Final separation and purification is done by any of the distillation or solvent extraction processes used for BTX aromatics (benzene, toluene, and xylene isomers).[15]
Other preparative routes
Toluene can be prepared by a variety of methods. For example, benzene reacts with methanol in presence of a solid acid to give toluene and water:[15]
Uses
Summarize
Perspective
Toluene is one of the most abundantly produced chemicals. Its main uses are (1) as a precursor to benzene and xylenes, (2) as a solvent for thinners, paints, lacquers, adhesives, and (3) as an additive for gasoline.[15] In 2013, worldwide sales of toluene amounted to about 24.5 billion US dollars.[38]
Precursor to benzene and xylenes
Toluene is converted to benzene via hydrodealkylation:
- C6H5CH3 + H2 → C6H6 + CH4
Its transalkylation gives a mixture of benzene and xylenes.
Solvent
Toluene is widely used in the paint, dye, rubber, chemical, glue, printing, and pharmaceutical industries as a solvent.[39] Nail polish, paintbrush cleaners, and stain removers may contain toluene. Manufacturing of explosives (TNT) uses it as well. Toluene is also found in cigarette smoke and car exhaust. If not in contact with air, toluene can remain unchanged in soil or water for a long time.[40]
Toluene is a common solvent, e.g. for paints, paint thinners, strippers, silicone sealants,[41] many chemical reactants, rubber, printing ink, adhesives (glues), lacquers, leather tanners, and disinfectants.[15]
Fuel
Toluene is an octane booster in gasoline fuels for internal combustion engines as well as jet fuel and turbocharged engines in Formula One.[42]
In Australia in 2003, toluene was found to have been illegally combined with petrol in fuel outlets for sale as standard vehicular fuel. Toluene incurs no fuel excise tax, while other fuels are taxed at more than 40%, providing a greater profit margin for fuel suppliers. The extent of toluene substitution is claimed to be 60%.[43][44]
Niche applications
In the laboratory, toluene is used as a solvent for carbon nanomaterials, including nanotubes and fullerenes, and it can also be used as a fullerene indicator. The color of the toluene solution of C60 is bright purple. Toluene is used as a cement for fine polystyrene kits (by dissolving and then fusing surfaces) as it can be applied very precisely by brush and contains none of the bulk of an adhesive. Toluene can be used to break open red blood cells in order to extract hemoglobin in biochemistry experiments. Toluene has also been used as a coolant for its good heat transfer capabilities in sodium cold traps used in nuclear reactor system loops. Toluene had also been used in the process of removing the cocaine from coca leaves in the production of Coca-Cola syrup.[45]
Remove ads
Toxicology and metabolism
Summarize
Perspective
The environmental and toxicological effects of toluene have been extensively studied.[46]
Toluene is irritating to the eyes, skin, and respiratory tract. It is absorbed slowly through the skin. It can cause systemic toxicity by inhalation or ingestion. Inhalation is the most common route of exposure. Symptoms of toluene poisoning include central nervous system effects (headache, dizziness, drowsiness, ataxia, euphoria, tremors, hallucinations, seizures, and coma), chemical pneumonitis, respiratory depression, ventricular arrhythmias, nausea, vomiting, and electrolyte imbalances.[39]
Inhalation of toluene in low to moderate levels can cause tiredness, confusion, weakness, drunken-type actions, memory loss, nausea, loss of appetite, hearing loss,[47][48][49] and colour vision loss.[50] Some of these symptoms usually disappear when exposure is stopped. Inhaling high levels of toluene in a short time may cause light-headedness, nausea, or sleepiness, unconsciousness, and even death.[51][52] Toluene is, however, much less toxic than benzene, and as a consequence, largely replaced it as an aromatic solvent in chemical preparation. The US Environmental Protection Agency (EPA) states that the carcinogenic potential of toluene cannot be evaluated due to insufficient information.[53]
Toluene occurs as an indoor air pollutant in a number of processes including electrosurgery, and can be removed from the air with an activated carbon filter.[54]
Similarly to many other solvents such as 1,1,1-trichloroethane and some alkylbenzenes, toluene has been shown to act as a non-competitive NMDA receptor antagonist and GABAA receptor positive allosteric modulator.[55] Additionally, toluene has been shown to display antidepressant-like effects in rodents in the forced swim test (FST) and the tail suspension test (TST),[55] likely due to its NMDA antagonist properties.
Toluene inhibits excitatory ion channels such as the NMDA receptor, nicotinic acetylcholine receptor, and the serotonin 5-HT3 receptor. It also potentiates the function of inhibitory ion channels, such as the GABAA and glycine receptors. In addition, toluene disrupts voltage-gated calcium channels and ATP-gated ion channels.[56]
Recreational use
Toluene is sometimes used as a recreational inhalant ("glue sniffing"), likely on account of its euphoric and dissociative effects,[55] in a manner unintended by manufacturers. People inhale toluene-containing products (e.g., paint thinner, contact cement, correction pens, model glue, etc.) for its intoxicating effect. The possession and use of toluene and products containing it are regulated in many jurisdictions, for the supposed reason of preventing minors from obtaining these products for recreational drug purposes. As of 2007, 24 US states had laws penalizing use, possession with intent to use, and/or distribution of such inhalants.[57] In 2005 the European Union banned the general sale of products consisting of greater than 0.5% toluene.[58]
Bioremediation
Several types of fungi including Cladophialophora, Exophiala, Leptodontidium (syn. Leptodontium), Pseudeurotium zonatum, and Cladosporium sphaerospermum, and certain species of bacteria can degrade toluene, using it as a source of carbon and energy.[59]
Remove ads
References
Cited sources
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads








![{\displaystyle {\mathrm {C} {\vphantom {A}}_{\smash[{t}]{6}}\mathrm {H} {\vphantom {A}}_{\smash[{t}]{6}}{}+{}\mathrm {CH} {\vphantom {A}}_{\smash[{t}]{3}}\mathrm {OH} {}\mathrel {\xrightarrow {\mathrm {t} {\vphantom {A}}^{o}} } {}\mathrm {C} {\vphantom {A}}_{\smash[{t}]{6}}\mathrm {H} {\vphantom {A}}_{\smash[{t}]{5}}\mathrm {CH} {\vphantom {A}}_{\smash[{t}]{3}}{}+{}\mathrm {H} {\vphantom {A}}_{\smash[{t}]{2}}\mathrm {O} }}](http://wikimedia.org/api/rest_v1/media/math/render/svg/b6cd1c2e0452714547fc7f9b995f21c2ab391336)