Top Qs
Timeline
Chat
Perspective
Caesium auride
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Caesium auride is the inorganic compound with the formula CsAu. It is the Cs+ salt of the unusual Au− anion.[2]
Remove ads
Preparation and reactions
CsAu is obtained by heating a stoichiometric mixture of caesium and gold. The two metallic-yellow liquids react to give a transparent yellow product.[3] Despite being a compound of two metals, CsAu lacks metallic properties since it is a salt with localized charges; it instead behaves as a semiconductor with band gap 2.6 eV.[4]
The compound hydrolyzes readily, yielding caesium hydroxide, metallic gold, and hydrogen.[3]
- 2 CsAu + 2 H2O → 2 CsOH + 2 Au + H2
The solution in liquid ammonia is brown, and the ammonia adduct CsAu·NH3 is blue; the latter has ammonia molecules intercalated between layers of the CsAu crystal parallel to the (110) plane. Solutions undergo metathesis with tetramethylammonium loaded ion exchange resin to give tetramethylammonium auride.[3]
Remove ads
Crystal structure
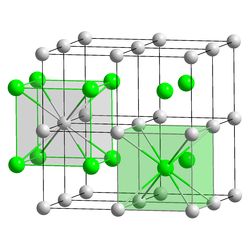
Caesium auride has a cubic lattice structure of the CsCl type. Each caesium atom is octahedrally coordinated with 8 gold atoms, and vice versa. The lattice constant at ambient conditions is approximately 4.24 Å, close to that of CsCl but slightly larger due to the larger Au−
ionic radius compared to Cl−
. The bonding is predominantly ionic,[5] as found by X-ray photoelectron spectroscopy, because gold has a much higher electronegativity than caesium.
Remove ads
References
Further reading
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads