Top Qs
Timeline
Chat
Perspective
Caesium azide
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Caesium azide or cesium azide is an inorganic compound of caesium and nitrogen. It is a salt of azide with the formula CsN3.
Remove ads
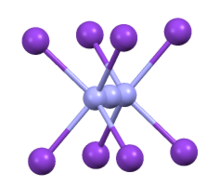
Structure
CsN3 adopts the same structure as KN3, RbN3, and TlN3, crystallizing in a tetragonal distorted caesium chloride structure where each azide ion coordinates to eight metal cations, and each metal cation coordinates to eight terminal N centers.[2] When heated to 151 °C, it transitions to a cubic structure.[3]
Preparation and reactions
Caesium azide can be prepared from the neutralization reaction between hydrazoic acid and caesium hydroxide:[4]
CsOH + HN3 → CsN3 + H2O
Caesium carbonate can also be used as the base:
Cs2CO3 + HN3 → CsN3 + CO2 + H2O
Caesium sulfate reacts with barium azide to form insoluble barium sulfate and caesium azide:
Cs2SO4 + Ba(N3)2 → 2CsN3 + BaSO4↓
The thermal decomposition of CsN3 in vacuo can be used as a method of generating high purity caesium metal:[5]
2 CsN3 → 2 Cs + 3 N2
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads