Top Qs
Timeline
Chat
Perspective
Caesium hydride
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Caesium hydride or cesium hydride is an inorganic compound of caesium and hydrogen with the chemical formula CsH. It is an alkali metal hydride. It was the first substance to be created by light-induced particle formation in metal vapor,[2] and showed promise in early studies of an ion propulsion system using caesium.[3] It is the most reactive stable alkaline metal hydride of all. It is a powerful superbase and reacts with water extremely vigorously.
The caesium nucleus in CsH can be hyperpolarized through interactions with an optically pumped caesium vapor in a process known as spin-exchange optical pumping (SEOP). SEOP can increase the nuclear magnetic resonance (NMR) signal of caesium nucleus by an order of magnitude.[4]
It is very difficult to make caesium hydride in a pure form. Caesium hydride can be produced by heating caesium carbonate and metallic magnesium in hydrogen at 580 to 620 °C.[5]
Remove ads
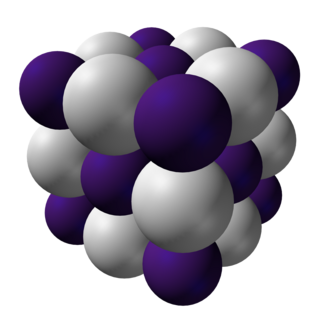
Crystal structure
At room temperature and atmospheric pressure, CsH has the same structure as NaCl.
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads