Top Qs
Timeline
Chat
Perspective
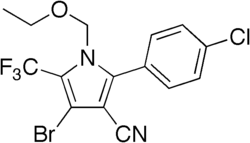
Chlorfenapyr
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Chlorfenapyr is an insecticide, and specifically a pro-insecticide (meaning it is metabolized into an active insecticide after entering the host).[1] It is derived from a class of microbially produced compounds known as halogenated pyrroles.
Remove ads
History and Applications
Chlorfenapyr was developed by American Cyanamid from the natural product dioxapyrrolomycin, which was isolated from Streptomyces fumanus.[2]
The United States Environmental Protection Agency initially denied registration in 2000 for use on cotton primarily because of concerns that the insecticide was toxic to birds and because effective alternatives were available.[3] However, it was registered by the EPA in January, 2001 for use on non-food crops in greenhouses.[4] In 2005, the EPA established a tolerance for residues of chlorfenapyr in or on all food commodities.
Chlorfenapyr is also used as a wool insect-proofing agent, and was introduced as an alternative to synthetic pyrethroids due to a lower toxicity to mammalian and aquatic life.[5]
In April 2016, in Pakistan, 31 people died when their food was spiked with chlorfenapyr.[6]
Remove ads
Mode of Action
Chlorfenapyr works by disrupting the production of adenosine triphosphate, specifically, "Oxidative removal of the N-ethoxymethyl group of chlorfenapyr by mixed function oxidases forms the compound CL 303268. CL 303268 uncouples oxidative phosphorylation at the mitochondria, resulting in disruption of production of ATP, cellular death, and ultimately organism mortality."[1] It is in IRAC group 13.
Remove ads
Notes
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads