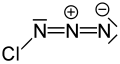Top Qs
Timeline
Chat
Perspective
Chlorine azide
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Chlorine azide (ClN3) is an inorganic compound that was discovered in 1908 by Friedrich Raschig.[2] Concentrated ClN3 is notoriously unstable and may spontaneously detonate at any temperature.[3]
Remove ads
Preparation and reactions
Chlorine azide is prepared by passing chlorine gas over silver azide, or by an addition of acetic acid to a solution of sodium hypochlorite and sodium azide.[4]
Chlorine azide further reacts with silver azide to produce a very unstable allotrope of nitrogen, hexanitrogen (N6), which decomposes to dinitrogen above 80 K (−193.2 °C; −315.7 °F).[5]
Explosive characteristics
Chlorine azide is extremely sensitive. It may explode, sometimes even without apparent provocation; it is thus too sensitive to be used commercially unless first diluted in solution. Chlorine azide reacts explosively with 1,3-butadiene, ethane, ethene, methane, propane, phosphorus, silver azide, and sodium. On contact with acid, chlorine azide decomposes, evolving toxic and corrosive hydrogen chloride gas.[6]
Remove ads
Regulatory information
Its shipment is subject to strict reporting requirements and regulations by the US Department of Transportation.
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads