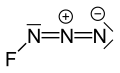Top Qs
Timeline
Chat
Perspective
Fluorine azide
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Fluorine azide or triazadienyl fluoride is a yellow green gas composed of nitrogen and fluorine with formula FN3.[1] Its properties resemble those of ClN3, BrN3, and IN3.[2] The bond between the fluorine atom and the nitrogen is very weak, leading to this substance being very unstable and prone to explosion.[3] Calculations show the F–N–N angle to be around 102° with a straight line of 3 nitrogen atoms.[4]
The gas boils at –30° and melts at –139 °C.[5]
It was first made by John F. Haller in 1942.[6]
Remove ads
Reactions
Fluorine azide can be made by reacting hydrazoic acid or sodium azide, with fluorine gas.[5][7]
- HN3 + F2 → N3F + HF
- NaN3 + F2 → N3F + NaF
Fluorine azide decomposes without explosion at normal temperatures to make dinitrogen difluoride:
- 2 FN3 → N2F2 + 2 N2.[1]
At higher temperatures such as 1000 °C fluorine azide breaks up into nitrogen monofluoride radical:[7]
- FN3 → NF + N2
The FN itself dimerizes on cooling.
- 2 NF → N2F2
Solid or liquid FN3 can explode, releasing a large amount of energy. A thin film burns at the rate of 1.6 km/s.[8] Due to the explosion hazard, only very small quantities of this substance should be handled at a time.[9]
FN3 adducts can be formed with the Lewis acids boron trifluoride (BF3) and arsenic pentafluoride (AsF5) at -196 °C. These molecules bond with the first nitrogen atom from the fluorine.[10]
Remove ads
Properties
Spectroscopy
| Parameter | Value[9] | Unit |
| A | 48131.448 | MHz |
| B | 5713.266 | MHz |
| C | 5095.276 | MHz |
| μa | 1.1 | |
| μb | 0.7 | |
Shape
Distances between atoms are F–N 0.1444 nm, FN=NN 0.1253 nm and FNN=N 0.1132 nm.[9]
Physical
FN3 has a density of 1.3 g/cm3.[11]
FN3 adsorbs on to solid surfaces of potassium fluoride, but not onto lithium fluoride or sodium fluoride. This property was being investigated so that FN3 could boost the energy of solid propellants.[11]
The ultraviolet photoelectric spectrum shows ionisation peaks at 11.01, 13,72, 15.6, 15.9, 16.67, 18.2, and 19.7 eV. Respectively these are assigned to the orbitals: π, nN or nF, nF, πF, nN or σ, π and σ.[3]
Remove ads
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads