Top Qs
Timeline
Chat
Perspective
Cobalt tetracarbonyl hydride
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Cobalt tetracarbonyl hydride is an organometallic compound with the formula HCo(CO)4. It is a volatile, yellow liquid that forms a colorless vapor and has an intolerable odor.[2] The compound readily decomposes upon melt and in absentia of high CO partial pressures forms Co2(CO)8. Despite operational challenges associated with its handling, the compound has received considerable attention for its ability to function as a catalyst in hydroformylation. In this respect, HCo(CO)4 and related derivatives have received significant academic interest for their ability to mediate a variety of carbonylation (introduction of CO into inorganic compounds) reactions.
Remove ads
Structure and properties
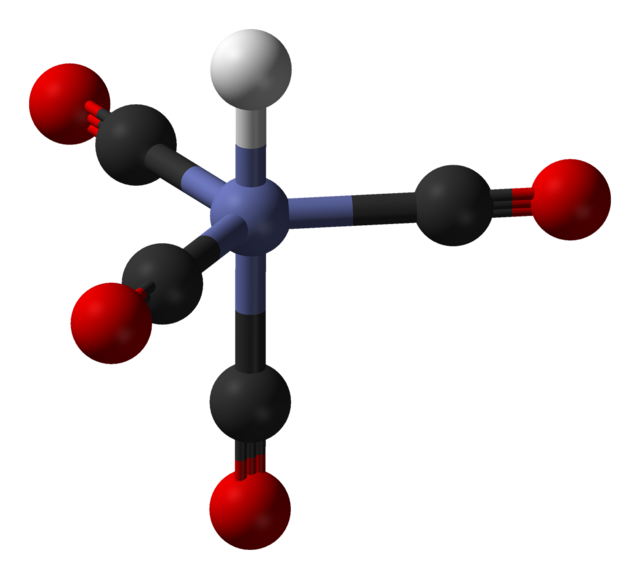
HCo(CO)4 adopts trigonal bipyramidal structure, with the hydride ligand occupying one of the axial positions, giving an overall symmetry of C3v. The three equatorial CO ligands are slightly bent out of the equatorial plane.[6] The Co–CO and Co–H bond distances were determined by gas-phase electron diffraction to be 1.764 and 1.556 Å, respectively.[7] Assuming the presence of a formal hydride ion, the oxidation state of cobalt in this compound is +1.
But unlike some other transition-metal hydrides complexes, HCo(CO)4 is highly acidic, with a pKa of 8.5.[8] It readily undergoes substitution by tertiary phosphines and other Lewis-bases. For example, triphenylphosphine gives HCo(CO)3PPh3 and HCo(CO)2(PPh3)2. These derivatives are more stable than HCo(CO)4 and are used industrially to improve catalyst selectivity in hydroformylation.[9] These derivatives are generally less acidic than HCo(CO)4.[8]
The compound decomposes easily. It is colorless when pure, but, soon after melting, develops a yellow tinge due to decomposition to cobalt tetracarbonyl dimer.[3]
Remove ads
Preparation
Tetracarbonylhydrocobalt was first described by Hieber in the early 1930s.[10] It was the second transition metal hydride to be discovered, after H2Fe(CO)4. Laboratory samples are prepared typically from the Brønsted conjugate base, Co(CO)−
4. The latter can be produced from direct carbonylation of cobaltous salts in base,[3] possibly with a cysteine catalyst...[11]
- 2 Co(NO3)2 + 11 CO + 12 KOH → 2 KCo(CO)4 + 4 KNO3 + 3 K2CO3 + 6 H2O
...or applying a reducing agent like sodium amalgam to Co2(CO)8, which gives sodium tetracarbonylcobaltate:[6]
- Co2(CO)8 + 2 Na → 2 NaCo(CO)4
- NaCo(CO)4 + H+ → HCo(CO)4 + Na+
Since HCo(CO)4 decomposes so readily, it is usually generated in situ by hydrogenation of Co2(CO)8.[9][12]
- Co2(CO)8 + H2 ⇌ 2 HCo(CO)4
The thermodynamic parameters for the equilibrium reaction were determined by infrared spectroscopy to be ΔH = 4.054 kcal mol−1, ΔS = −3.067 cal mol−1 K−1.[9]
Remove ads
Applications
Tetracarbonylhydridocobalt was the first transition metal hydride to be used in industry.[13] In 1953 evidence was disclosed that it is the active catalyst for the conversion of alkenes, CO, and H2 to aldehydes, a process known as hydroformylation (oxo reaction).[14] Although the use of cobalt-based hydroformylation has since been largely superseded by rhodium-based catalysts, the world output of C3–C18 aldehydes produced by tetracarbonylhydrocobalt catalysis is about 100,000 tons per year, roughly 2% of the total.[13]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads

