Top Qs
Timeline
Chat
Perspective
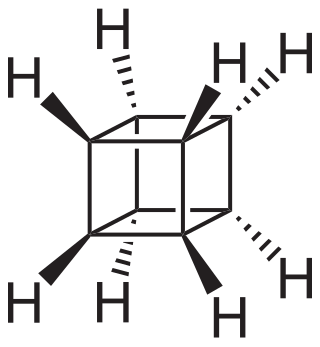
Cubane
Organic compound (C8H8) with a cube carbon structure From Wikipedia, the free encyclopedia
Remove ads
Cubane is a synthetic hydrocarbon compound with the formula C8H8. It consists of eight carbon atoms arranged at the corners of a cube, with one hydrogen atom attached to each carbon atom. A solid crystalline substance, cubane is one of the Platonic hydrocarbons and a member of the prismanes. It was first synthesized in 1964 by Philip Eaton and Thomas Cole.[4] Before this work, Eaton believed that cubane would be impossible to synthesize due to the "required 90 degree bond angles".[5][6] The cubic shape requires the carbon atoms to adopt an unusually sharp 90° bonding angle, which would be highly strained as compared to the 109.45° angle of a tetrahedral carbon. Once formed, cubane is quite kinetically stable, due to a lack of readily available decomposition paths. It is the simplest hydrocarbon with octahedral symmetry.
Having high potential energy and kinetic stability makes cubane and its derivative compounds useful for controlled energy storage. For example, octanitrocubane and heptanitrocubane have been studied as high-performance explosives. These compounds also typically have a very high density for hydrocarbon molecules. The resulting high energy density means a large amount of energy can be stored in a comparably smaller amount of space, an important consideration for applications in fuel storage and energy transport. Furthermore, their geometry and stability make them suitable isosteres for benzene rings.[7]
Remove ads
Synthesis
Summarize
Perspective
The classic 1964 synthesis starts with the conversion of 2-cyclopentenone to 2-bromocyclopentadienone:[4][8]
Allylic bromination with N-bromosuccinimide in carbon tetrachloride followed by addition of molecular bromine to the alkene gives a 2,3,4-tribromocyclopentanone. Treating this compound with diethylamine in diethyl ether causes elimination of two equivalents of hydrogen bromide to give the diene product.

The construction of the eight-carbon cubane framework begins when 2-bromocyclopentadienone undergoes a spontaneous Diels-Alder dimerization. One ketal of the endo isomer is subsequently selectively deprotected with aqueous hydrochloric acid to 3.
In the next step, the endo isomer 3 (with both alkene groups in close proximity) forms the cage-like isomer 4 in a photochemical [2+2] cycloaddition. The bromoketone group is converted to ring-contracted carboxylic acid 5 in a Favorskii rearrangement with potassium hydroxide. Next, the thermal decarboxylation takes place through the acid chloride (with thionyl chloride) and the tert-butyl perester 6 (with tert-butyl hydroperoxide and pyridine) to 7; afterward, the acetal is once more removed in 8. A second Favorskii rearrangement gives 9, and finally another decarboxylation gives, via 10, cubane (11).
A more approachable laboratory synthesis of disubstituted cubane involves bromination of the ethylene ketal of cyclopentanone to give a tribromocyclopentanone derivative. Subsequent steps involve dehydrobromination, Diels-Alder dimerization, etc.[9][10]

The resulting cubane-1,4-dicarboxylic acid is used to synthesize other substituted cubanes. Cubane itself can be obtained nearly quantitatively by photochemical decarboxylation of the thiohydroxamate ester (the Barton decarboxylation).[11]
Remove ads
Reactions and derivatives
Summarize
Perspective
Cubane is highly strained, but cannot decompose because the resultant cubene molecules are pyramidal alkenes, too high-energy for most elimination pathways. Certain metallic ions catalyze σ-bond rearrangement to cuneane:[12][13]
With a rhodium catalyst, cubane first forms syn-tricyclooctadiene, which can thermally decompose to cyclooctatetraene at 50–60 °C.[14]
The main cubane functionalization challenge is C-H bond activation. Cubenes still inhibit decomposition during radical substitution, but the reaction offers little control against oversubstitution. In polar reactions, cubane reacts somewhat similarly to arenes or other cluster compounds: it metallates easily.[15] Cubane is slightly acidic, deprotonating about 63000 times faster than cyclohexane.[16]
Cubane substituents generally display normal reactivity. For example a Curtius rearrangement followed by organic oxidation converts cubane tetra(carbonylchloride) to tetranitrocubane.[15] However, electron-rich substituents such as alcohols can enable decomposition; they stabilize the cubene intermediate as a ketone (or equivalent) tautomer.[17]
Oxalyl chloride oxidizes cubane carboxamides to give a norcubane-furanone derivative.[18]
Hypercubane was predicted to exist in a 2014 publication.[19][20]
Persubstituted derivatives
Octaphenylcubane pre-dates the parent compound. Freedman synthesized it from tetraphenylcyclobutadiene nickel bromide in 1962. It is a sparingly soluble colourless compound that melts at 425–427 °C.[3][21][22][23]
Octanitrocubane is a green explosive.
Both heptafluorocubane and octafluorocubane were synthesized in 2022 to study octafluorocubane's unusual electronic structure.[24] Single-electron reduction to the radical anion C
8F−
8 traps[25] an otherwise-free electron inside the cube, making it the world's smallest box.[26]
Cubenes and poly-cubylcubane
Despite their orbital strain, two cubenes have been synthesized, and a third analyzed computationally. ortho-Cubene, produced via lithium-halogen exchange followed by elimination,[17] was the most pyramidalized alkene ever made at the time of its synthesis;[27] meta-cubene is even less stable, and para-cubene probably only exists as a diradical rather than an actual diagonal bond.[28] They rapidly undergo nucleophilic addition.[29]
Decomposition of cubenes has enabled chemists to synthesize cubylcubane, as well as higher oligomers.[29] Per X-ray diffraction, the central cubane-cubane bond is exceedingly short (1.458 Å), much shorter than the typical C-C single bond (1.578 Å). This is attributed to the fact that the exocyclic orbitals of cubane are s-rich and close to the nucleus.[30]
The oligo-cubylcubanes are rigid molecular rods considered for liquid crystal design, but scarcely accessible through conventional organic synthesis. Absent solubizing groups on the cubane monomer, oligomers with at least 4 units are essentially insoluble.[31] Poly-cubylcubane is, however, synthesizable via high pressure, solid-state polymerization. It exhibits exceptionally high refractive index.[32]
Remove ads
See also
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads