Top Qs
Timeline
Chat
Perspective
Cymene
From Wikipedia, the free encyclopedia
Remove ads
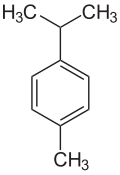
Cymene describes organic compounds with the formula CH3C6H4CH(CH3)2. Three isomers exist: 1,2- 1,3-, and 1,4-. All are colorless liquids, immiscible in water, with similar boiling points. They are classified as aromatic hydrocarbons. They bear two substituents: an isopropyl (CH(CH3)2) group and a methyl group.[1]
Remove ads
Production and reactions
m- and p-Cymene are prepared by alkylation of toluene with propylene:
- CH3C6H5 + 2 CH3CH=CH2 → CH3C6H4CH(CH3)2
These alkylations are catalyzed by various Lewis acids, such as aluminium trichloride.
m- and p-Cymene are mainly of interest as precursors to the respective cresols, which exploits the Hock rearrangements.[1]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads