Top Qs
Timeline
Chat
Perspective
Polymerase
Class of enzymes which synthesize nucleic acid chains or polymers From Wikipedia, the free encyclopedia
Remove ads
In biochemistry, a polymerase is an enzyme (EC 2.7.7.6/7/19/48/49) that synthesizes long chains of polymers or nucleic acids. DNA polymerase and RNA polymerase are used to assemble DNA and RNA molecules, respectively, by copying a DNA template strand using base-pairing interactions or half ladder replication.
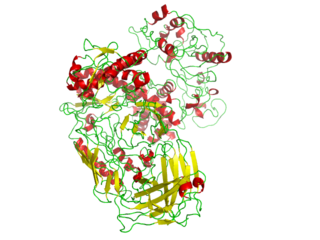
A DNA polymerase from the thermophilic bacterium, Thermus aquaticus (Taq) (PDB 1BGX, EC 2.7.7.7), is used in the polymerase chain reaction, an important technique of molecular biology.
A polymerase may be template-dependent or template-independent. Poly-A-polymerase is an example of template independent polymerase. Terminal deoxynucleotidyl transferase is also known to have template independent and template dependent activities.
Remove ads
By function
- DNA polymerase (DNA-directed DNA polymerase, DdDP)
- Family A: DNA polymerase I; Pol γ, θ, ν
- Family B: DNA polymerase II; Pol α, δ, ε, ζ
- Family C: DNA polymerase III holoenzyme
- Family X: Pol β, λ, μ
- Terminal deoxynucleotidyl transferase (TDT), which lends diversity to antibody heavy chains.[1]
- Family Y: DNA polymerase IV (DinB) and DNA polymerase V (UmuD'2C) - SOS repair polymerases; Pol η, ι, κ
- Reverse transcriptase (RT; RNA-directed DNA polymerase; RdDP)
- DNA-directed RNA polymerase (DdRP, RNAP)
- Multi-subunit (msDdRP): RNA polymerase I, RNA polymerase II, RNA polymerase III
- Single-subunit (ssDdRP): T7 RNA polymerase, POLRMT
- Primase, PrimPol
- RNA replicase (RNA-directed RNA polymerase, RdRP)
- Viral (single-subunit)
- Eukaryotic cellular (cRdRP; dual-subunit)
- Template-less RNA elongation
Remove ads
By structure
Polymerases are generally split into two superfamilies, the "right hand" fold (InterPro: IPR043502) and the "double psi beta barrel" (often simply "double-barrel") fold. The former is seen in almost all DNA polymerases and almost all viral single-subunit polymerases; they are marked by a conserved "palm" domain.[2] The latter is seen in all multi-subunit RNA polymerases, in cRdRP, and in "family D" DNA polymerases found in archaea.[3][4] The "X" family represented by DNA polymerase beta has only a vague "palm" shape, and is sometimes considered a different superfamily (InterPro: IPR043519).[5]
Primases generally don't fall into either category. Bacterial primases usually have the Toprim domain, and are related to topoisomerases and mitochondrial helicase twinkle.[6] Archae and eukaryotic primases form an unrelated AEP family, possibly related to the polymerase palm. Both families nevertheless associate to the same set of helicases.[7]
- Right hand structure of Bacteriophage RB69, a family B DdRP.
Remove ads
See also
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads

