Top Qs
Timeline
Chat
Perspective
Difluoride
Index of chemical compounds with the same name From Wikipedia, the free encyclopedia
Remove ads
Difluorides are chemical compounds with two fluorine atoms per molecule (or per formula unit).
| Order and disorder in difluorides | |
 |
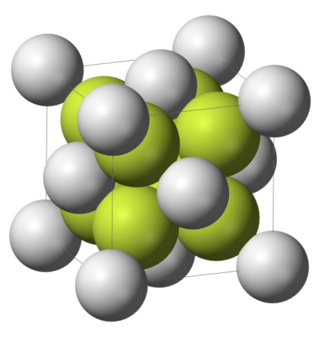
 |
| The fluorite structure | Beryllium fluoride glass |
Metal difluorides are all ionic. Despite being highly ionic, the alkaline earth metal difluorides generally have extremely high lattice stability and are thus insoluble in water. The exception is beryllium difluoride. In addition, many transition metal difluorides are water-soluble.
Calcium difluoride is a notable compound. In the form of the mineral fluorite it is the major source of commercial fluorine. It also has an eponymic crystal structure, which is an end member of the spectrum starting from bixbyite and progressing through pyrochlore.
Remove ads
List of the difluorides
Summarize
Perspective
Examples of the difluorides include:
Alkaline earth metal difluorides
The alkaline earth metals all exhibit the oxidation state +2, and form difluorides. The difluoride of radium is however not well established due to the element's high radioactivity.
- Beryllium difluoride
- Magnesium fluoride
- Calcium fluoride
- Strontium difluoride
- Barium fluoride
- Radium fluoride
Lanthanide difluorides
- Neodymium difluoride[citation needed]
- Samarium difluoride[5]
- Europium difluoride[5]
- Dysprosium difluoride[citation needed]
- Thulium difluoride[5]
- Ytterbium difluoride[6][5]
Transition metal difluorides
Compounds of the form MF2:
Post-transition metal difluorides
Nonmetal and metalloid difluorides
Noble gas difluorides
- Helium difluoride (hypothetical) [7]
- Argon difluoride (predicted)
- Krypton difluoride
- Xenon difluoride
- Radon difluoride
Bifluorides
The bifluorides contain the two fluorine atoms in a covalently bound HF2− polyatomic ion rather than as F− anions.
Organic difluorides
- Ethanedioyl difluoride
- Ethylidene difluoride
- Carbonyl difluoride
- Carbon dibromide difluoride (dibromodifluoromethane)
- Carbon dichloride difluoride (dichlorodifluormethane)
- Methyl difluoride
- Methylphosphonyl difluoride
- Polyvinylidene difluoride
Remove ads
References
Bibliography
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads