Top Qs
Timeline
Chat
Perspective
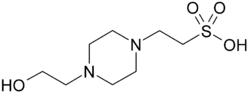
HEPES
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) is a zwitterionic sulfonic acid buffering agent. It is one of the twenty Good's buffers. HEPES is widely used in cell culture, largely because it is better at maintaining physiological pH despite changes in carbon dioxide concentration (produced by aerobic respiration) when compared to bicarbonate buffers, which are also commonly used in cell culture. [2] Lepe-Zuniga et al. reported an unwanted photochemical process wherein HEPES catalyzes a reaction with riboflavin when exposed to ambient light to produce hydrogen peroxide.[3][4] This is not a problem in bicarbonate-based cell culture buffers. It is therefore strongly advised to keep solutions containing both HEPES and riboflavin in darkness as much as possible to prevent oxidation.
HEPES has the following characteristics:
- pKa1 (25 °C) = 3
- pKa2 (25 °C) = 7.5
- Useful pH range = 2.5 to 3.5 or 6.8 to 8.2
HEPES has negligible metal ion binding,[5] making it a good choice as a buffer for enzymes which might be inhibited by metal chelation.
Remove ads
See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads