Top Qs
Timeline
Chat
Perspective
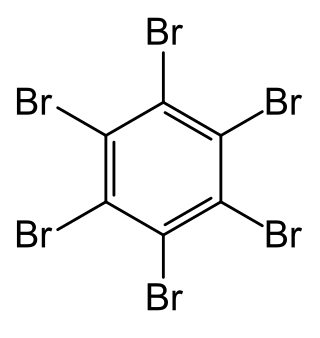
Hexabromobenzene
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Hexabromobenzene (HBB) is an organobromine compound with the formula C6Br6. It features a central benzene ring with six bromine substituents. Hexabromobenzene is a white powder that is not soluble in water but is soluble in ethanol, ether, and benzene. Its bromine content is above 86%.[5]
Remove ads
Preparation
It can be prepared by the reaction of benzene with 6 equivalents of bromine (Br2) in the presence of heat and UV light:[citation needed]
- C6H6 + 6 Br2 → C6Br6 + 6 HBr
The reaction produces six equivalents of hydrogen bromide.
Uses
Hexabromobenzene has seen use in high voltage capacitors as a flame retardant.[6]
Hexabromobenzene finds extensive use as a fire retardant additive in a range of materials including plastics, paper, and electrical goods, where it serves as a top-tier flame retardant. It was introduced to replace traditional organobromine fire retardants such as polybrominated derivatives of diphenyl ethers and biphenyls.[7] With a high melting point of 327 °C and a high bromide content of 86%, HBB significantly enhances the fire safety of these materials.[8] Iits widespread application also leads to its dispersion in the environment.
Remove ads
Metabolism
Hexabromobenzene was used in a study investigating its metabolic fate in female rats, wherein the substance was orally administered at doses of 16.6 mg/kg body weight every other day for a span of 2 weeks. Analysis of the rats' excreta revealed the presence of various metabolites, including unchanged HBB, pentabromobenzene, as well as oxygen- and sulfur-containing compounds.[9]
Dangers
Hexabromobenzene poses significant dangers due to its toxicity profile as classified by the GHS (Globally Harmonized System of Classification and Labeling of Chemicals). Classified as GHS07, HBB exhibits acute toxicity via oral, dermal, and inhalation routes, categorizing it under category 4 for this hazard. Additionally, it induces skin and eye irritation, classified under category 2 for both. Moreover, HBB is known to cause skin sensitization (category 1) and specific target organ toxicity upon single exposure (category 3), with the respiratory system being the primary target organ.[10]
The acute toxicity of brominated benzenes decreases with an increase in the number of bromine atoms in the molecule.[11] However, the potential for necrotic changes varies based on the position of these bromine atoms within the molecule. There are severe health risks associated with HBB exposure, warranting careful handling and stringent safety measures in its use and management.[10]
Remove ads
See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads