Top Qs
Timeline
Chat
Perspective
Homocysteine
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
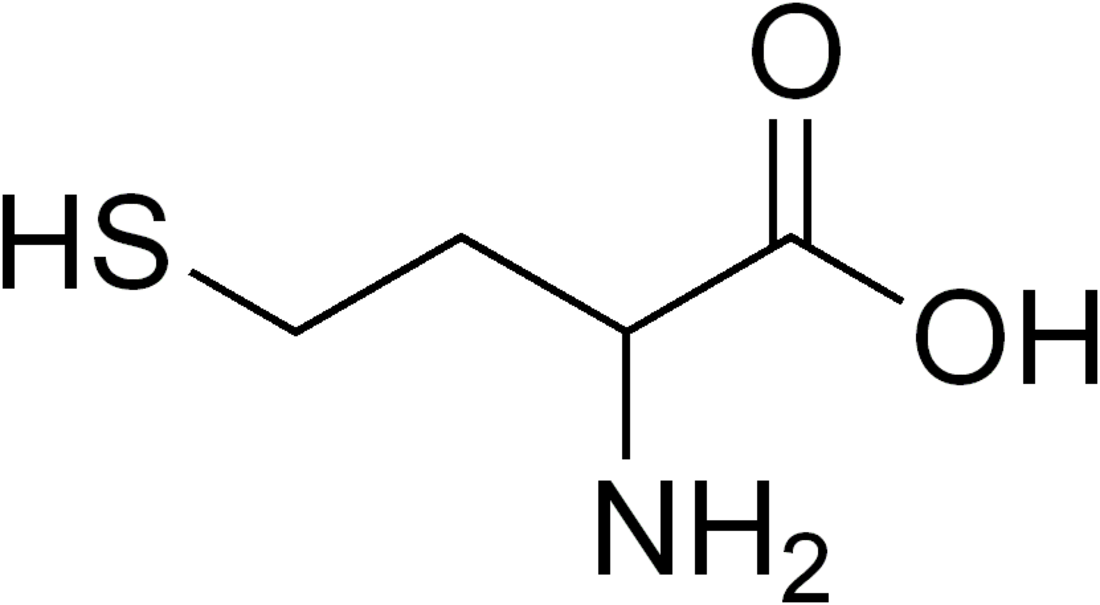
Homocysteine (/ˌhoʊmoʊˈsɪstiːn/; symbol Hcy) is a non-proteinogenic α-amino acid. It is a homologue of the amino acid cysteine, differing by an additional methylene bridge (−CH2−). It is biosynthesized from methionine by the removal of its terminal Cε methyl group.
Although the production of homocysteine is a normal part of the metabolism of methionine, an excess of homocysteine can be harmful. There are two primary ways for organisms such as humans to metabolize homocysteine: remethylation and transsulfuration.
Remethylation adds a methyl group to the homocysteine molecule, converting homocysteine back into methionine. There are two known remethylation pathways. One pathway requires vitamin B9 (folate) and B12 (cobalamin), which drive the MTR (methionine synthase) and MTRR (methionine synthase reductase) enzymes. The other pathway uses TMG (trimethylglycine) to drive the BHMT (betaine-homocysteine methyltransferase) enzyme.
Transsulfuration converts homocysteine to cystathionine. This pathway requires vitamin B6 to drive the CBS (cystathionine beta synthase) enzyme.[3] Cystathionine is the immediate precursor of the amino acid cysteine, which (along with glutamate and glycine), is incorporated into the tripeptide glutathione, a major antioxidant in the human body.
Homocysteine is therefore an important metabolic substrate. However, excessive levels of homocysteine can result in hyperhomocysteinemia, which is regarded as an indicator of cardiovascular disease risk. Homocysteine likely contributes to atherogenesis, which can result in ischemic injury. Therefore, hyperhomocysteinemia is a possible risk factor for coronary artery disease. Coronary artery disease occurs when an atherosclerotic plaque blocks blood flow to the coronary arteries, which supply the heart with oxygenated blood.[4][5] Hyperhomocysteinemia has also been correlated with the occurrence of blood clots, heart attacks, and strokes, although it is unclear whether hyperhomocysteinemia is an independent risk factor for these conditions.[6] Hyperhomocysteinemia has also been associated with early-term spontaneous abortions[7] and with neural tube defects.[8]

Remove ads
Biosynthesis and biochemical roles
Summarize
Perspective

Homocysteine is biosynthesized naturally via a multi-step process.[9] First, methionine receives an adenosine group from ATP, a reaction catalyzed by S-adenosyl-methionine synthetase, to give S-adenosyl methionine (SAM). SAM is widely used source of methyl radicals as a cofactor for radical SAM enzymes. Transfer of the methyl group to an acceptor molecule gives S-adenosyl-homocysteine. Hydrolysis of this thioether gives L-homocysteine. L-Homocysteine reacts with tetrahydrofolate (THF) to give L-methionine.[clarification needed][10]
Biosynthesis of cysteine
Mammals biosynthesize the amino acid cysteine via homocysteine. Cystathionine β-synthase catalyses the condensation of homocysteine and serine to give cystathionine. This reaction uses pyridoxine (vitamin B6) as a cofactor. Cystathionine γ-lyase then converts this double amino acid to cysteine, ammonia, and α-ketobutyrate. Bacteria and plants rely on a different pathway to produce cysteine, relying on O-acetylserine.[11]

Methionine salvage
Homocysteine can be recycled into methionine. This process uses N5-methyl tetrahydrofolate as the methyl donor and cobalamin (vitamin B12)-related enzymes. More detail on these enzymes can be found in the article for methionine synthase.
Other reactions of biochemical significance
Homocysteine can cyclize to give homocysteine thiolactone, a five-membered heterocycle. Because of this "self-looping" reaction, homocysteine-containing peptides tend to cleave themselves by reactions generating oxidative stress.[12]
Homocysteine also acts as an allosteric antagonist at Dopamine D2 receptors.[13]
It has been proposed that both homocysteine and its thiolactone may have played a significant role in the appearance of life on the early Earth.[14]
Remove ads
Homocysteine levels
Summarize
Perspective

Homocysteine levels typically are higher in men than women, and increase with age.[15][16]
Common levels in Western populations are 10 to 12 μmol/L, and levels of 20 μmol/L are found in populations with low B-vitamin intakes or in the elderly (e.g., Rotterdam, Framingham).[17][18]
It is decreased with methyl folate trapping, where it is accompanied by decreased methylmalonic acid, increased folate, and a decrease in formiminoglutamic acid.[19] This is the opposite of MTHFR C677T mutations, which result in an increase in homocysteine.[citation needed]
| Sex | Age | Lower limit | Upper limit | Unit | Elevated | Therapeutic target |
| Female | 12–19 years | 3.3[20] | 7.2[20] | μmol/L | > 10.4 μmol/L or > 140 μg/dl | < 6.3 μmol/L[21] or < 85 μg/dL[21] |
| 45[22] | 100[22] | μg/dL | ||||
| >60 years | 4.9[20] | 11.6[20] | μmol/L | |||
| 66[22] | 160[22] | μg/dL | ||||
| Male | 12–19 years | 4.3[20] | 9.9[20] | μmol/L | > 11.4 μmol/L or > 150 μg/dL | |
| 60[22] | 130[22] | μg/dL | ||||
| >60 years | 5.9[20] | 15.3[20] | μmol/L | |||
| 80[22] | 210[22] | μg/dL | ||||
The ranges above are provided as examples only; test results always should be interpreted using the range provided by the laboratory that produced the result.
Remove ads
Elevated homocysteine
Abnormally high levels of homocysteine in the serum, above 15 μmol/L, are a medical condition called hyperhomocysteinemia.[23] This has been claimed to be a significant risk factor for the development of a wide range of diseases, in total more than 100[24] including thrombosis,[25] neuropsychiatric illness,[26][27][28][29] in particular dementia[30] and fractures.[31][32] It also is found to be associated with microalbuminuria (moderately increased albuminuria), which is a strong indicator of the risk of future cardiovascular disease and renal dysfunction.[33] Vitamin B12 deficiency, even when coupled with high serum folate levels, has been found to increase overall homocysteine concentrations as well.[34]
Typically, hyperhomocysteinemia is managed with vitamin B6, vitamin B9, and vitamin B12 supplementation.[35] However, supplementation with these vitamins does not appear to improve cardiovascular disease outcomes.[36]
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads