Top Qs
Timeline
Chat
Perspective
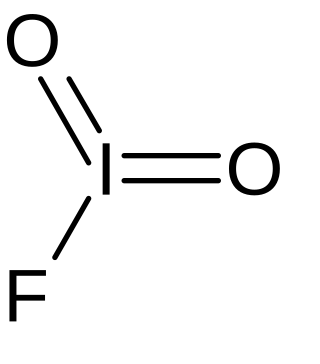
Iodyl fluoride
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Iodyl fluoride is an inorganic compound of iodine, fluorine, and oxygen with the chemical formula IO2F. It is in the form of colorless crystals. Iodyl fluoride features iodine in the oxidation state of +5. The compound was initially synthesized in 1951.[1]
Remove ads
Synthesis
Iodyl fluoride can be decomposed by iodosyl trifluoride heated to 110 °C (230 °F) in nitrogen. Since this reaction is reversible, the reaction requires constant removal of iodine pentafluoride.[1]
- 2 IOF3 ⇌ IO2F + IF5
It can also be obtained by dissolving iodine pentoxide, I2O5, in anhydrous hydrogen fluoride.[2]
- I2O5 + HF → IO2F + HIO3
Physical properties
Iodyl fluoride forms colorless crystals of orthorhombic system.[3] Reacts with water.[4]
Chemical properties
Iodyl fluoride is stable in dry air, but slowly hydrolyzes to iodic and hydrofluoric acids in moisture.[1]
- IO2F + H2O → HIO3 + HF
The compound reacts with strong fluorinating agents such as bromine trifluoride and selenium tetrafluoride to form iodine pentafluoride. Iodyl fluoride can be reduced to elemental iodine by pure hydrogen peroxide.[5][6]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads