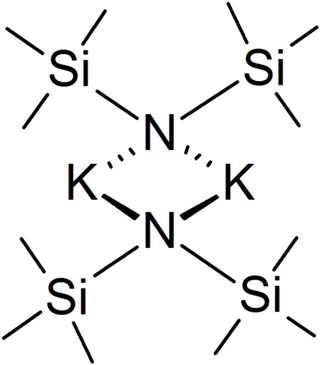Top Qs
Timeline
Chat
Perspective
Potassium bis(trimethylsilyl)amide
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Potassium bis(trimethylsilyl)amide (commonly abbreviated as KHMDS, Potassium(K) HexaMethylDiSilazide) or potassium hexamethyldisilazane[1] is the chemical compound with the formula ((CH3)3Si)2NK. It is a strong, non-nucleophilic base with an approximate pKa of 26 (compare to lithium diisopropylamide, at 36).[citation needed]
Remove ads
Structure
The methylsilyl groups give KHMDS good solubility in most organic solvents. Solution structures are either solvated monomers or dimers (or mixtures thereof) with this depending on the coordinating power, concentration, and temperature of the solvent.[3] In general, weakly coordinating solvents such as toluene and N,N-dimethylethylamine give dimers, where as THF and diglyme gave monomers at high dilution.[3] In the solid state, the unsolvated compound is dimeric, with two potassium and two nitrogen atoms forming a square.[4] KHMDS conducts electricity poorly in solution and in the melt, which is attributed to very strong ion pairing.
Remove ads
See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads