Top Qs
Timeline
Chat
Perspective
Lanthanum phosphide
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Lanthanum phosphide is an inorganic compound of lanthanum and phosphorus with the chemical formula LaP.
Remove ads
Synthesis
Lanthanum phosphide can be made by heating lanthanum metal with excess phosphorus in a vacuum:[2]
- 4 La + P4 → 4 LaP
Physical properties
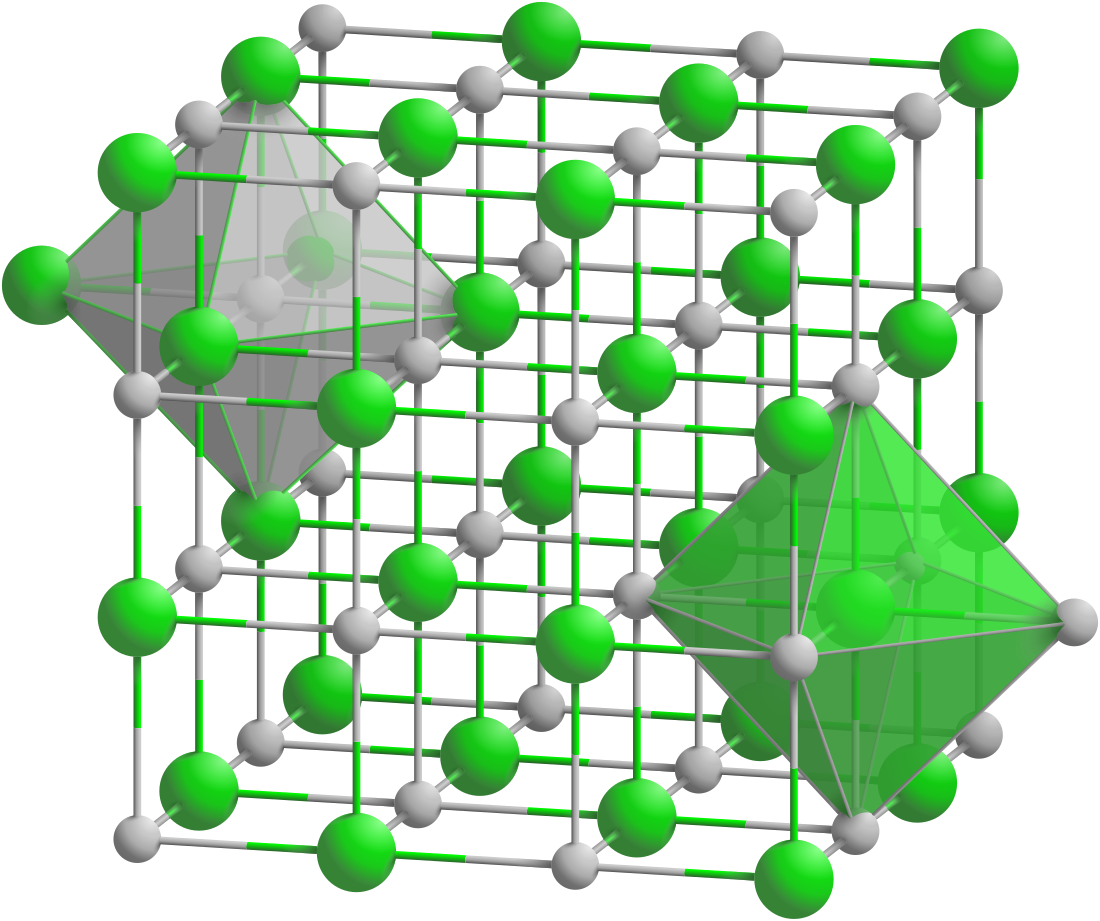
Lanthanum phosphide forms black crystals of a cubic system, space group Fm3m, cell parameters a = 0.6025 nm, with number of formulas per unit cell Z = 4.[1]
The crystals are very unstable and decompose in the open air.
Electronic properties
Lanthanum phosphide is an example of a strongly correlated material,[3] complicating theoretical prediction of its properties.
According to HSE06 calculations, lanthanum phosphide has been theoretically predicted to have an indirect band gap of 0.25 eV along the Γ-X direction.[4] According to HSE06 calculations with spin-orbit coupling, the band gap is predicted to be a direct gap of 0.72 eV at the X point.[5] Using EVGGA, the compound is predicted to have a band gap of 0.56 eV along the Γ-X direction.[6] FP-LAPW has predicted an indirect gap of 0.33 eV along the Γ-X direction.[3]
Chemical properties
Lanthanum phosphide reacts with water, releasing highly toxic phosphine gas:
- LaP + 3H2O → La(OH)3 + PH3
Uses
Lanthanum phosphide compound is a semiconductor used in high power, high frequency applications, and in laser diodes.[7][8]
Lanthanum polyphosphide
In addition to the simple phosphide, LaP, lanthanum and phosphorus can also form phosphorus-rich compounds such as LaP2[9] LaP5[10] and LaP7.[11]
References
Further reading
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads